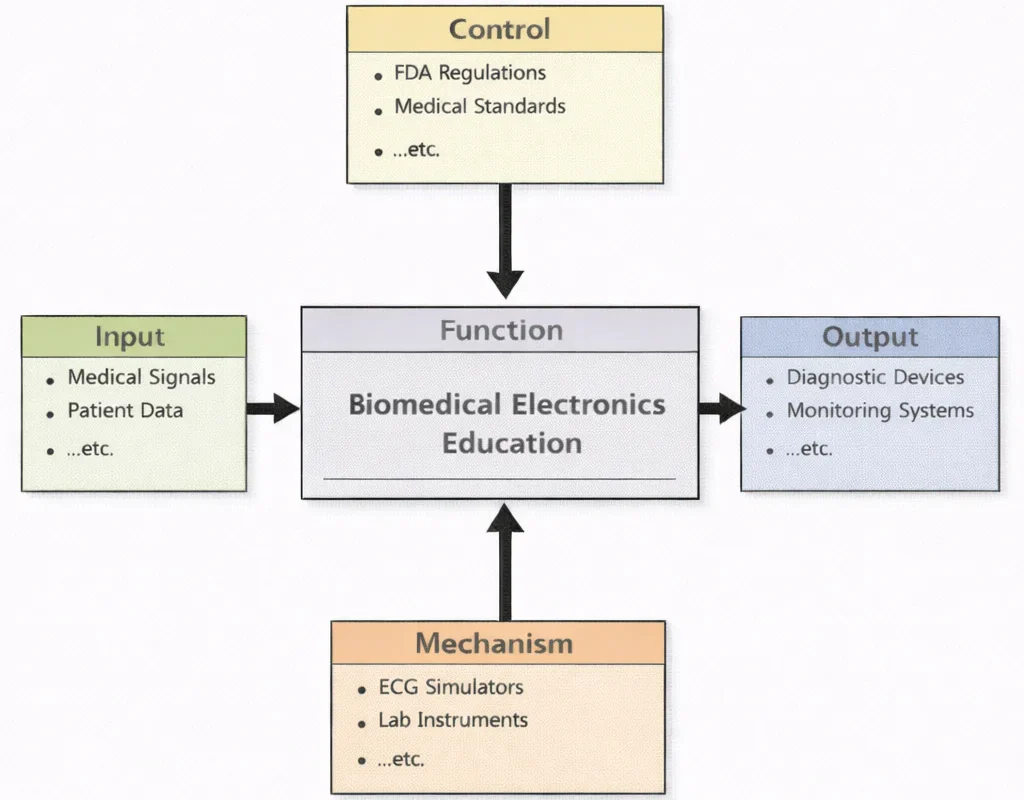
Biomedical Electronics Education, in essence, teaches students to make living systems “legible” to electronics—without losing respect for the fact that a patient is not a test bench. The diagram’s flow captures that discipline: real physiological inputs are translated into measurable, usable electrical forms; strict controls (safety, standards, ethics, and regulation) shape every design choice; and hands-on mechanisms (labs, tools, prototypes, teamwork) turn abstract theory into working practice. The output is not just the ability to wire a circuit, but the ability to build trustworthy biomedical devices—systems that acquire signals cleanly, reduce noise intelligently, protect users rigorously, and perform consistently in the messy reality of clinical environments.
Biomedical Electronics represents a transformative intersection between engineering and healthcare, offering life-enhancing solutions through innovation and precision. As a subfield of Electrical and Electronic Engineering, it harnesses principles from Electronics Engineering and advances in Embedded Systems and Microelectronics to build devices that monitor, diagnose, and treat medical conditions with remarkable accuracy.
A major area of development includes wearable and implantable devices, many of which rely on highly sensitive Instrumentation and Measurement systems. These systems, often integrated with Signal Processing algorithms, can extract critical health data from ECGs, oxygen sensors, or EEG monitors. Biomedical systems are increasingly interconnected through the Internet of Things (IoT) and Smart Technologies, enabling real-time updates to clinicians and even predictive diagnostics.
To maintain reliability and power autonomy, these devices benefit from research in Power Systems Engineering and the growing relevance of Renewable Energy and Energy Storage. Some implantable devices even explore the limits of miniaturized quantum effects, a field informed by Quantum Electronics. The future of assisted living and robotic surgery also lies within the reach of Robotics and Automation in E&E.
Control systems enable precision in life-support machines and prosthetic interfaces, drawing on fundamental principles from Control Systems Engineering. Furthermore, efficient communication of health metrics is critical in emergency care and telemedicine, supported by innovations in Communication Engineering.
Biomedical Electronics is also a contributor to environmentally conscious healthcare systems. Tools from Environmental Monitoring and Data Analysis help track hospital waste emissions and hygiene compliance. In line with global sustainability efforts, integration with Green Building and Sustainable Design ensures that medical facilities adopt energy-efficient systems.
Medical technology also intersects with efforts in Waste Management Engineering and Air Quality Engineering, especially in handling toxic disposables and maintaining sterile air environments. Broader policy implementation relies on Environmental Policy and Management and initiatives from Climate Change Mitigation and Adaptation.
In manufacturing, the deployment of biomedical devices depends on streamlined techniques developed in Industrial and Manufacturing Technologies. These production processes must align with the principles of Industrial Ecology and Circular Economy, ensuring safety, recyclability, and ethical sourcing. Increasingly, engineers are also partnering with specialists in Ecological Engineering to minimize ecological footprints of diagnostic equipment.
Finally, Biomedical Electronics contributes to public health resilience by supporting infrastructure in Renewable Energy Systems Engineering and ensuring water quality through Water Resources Engineering. For students preparing for university studies, this field offers a compelling mix of electrical principles, innovation, ethical responsibility, and social impact.

A futuristic clinical engineering workspace blends electronics and medicine: a large printed circuit board and components dominate the foreground, while a glowing 3D heart visualization and diagnostic dashboards (brain and imaging panels) fill the background. A clinician in a lab coat reviews instruments, another stands at a workstation, and nearby medical equipment and anatomical visuals suggest the design and testing of electronic systems used in monitoring, imaging, and life-support technologies.
Biomedical Electronics – Invisible FAQ
- What is biomedical electronics?
- Biomedical electronics applies electronic engineering to medical devices and systems that measure, monitor or influence physiological processes for diagnosis and treatment.
- How is biomedical electronics different from general electronics?
- General electronics targets consumer, industrial or communication uses, whereas biomedical electronics must meet strict requirements for patient safety, biocompatibility, regulatory compliance and accurate handling of physiological signals.
- What do biosensors and transducers do in biomedical devices?
- Biosensors and transducers convert biological or chemical quantities, such as glucose level or electrical activity of the heart, into electrical signals that can be amplified, processed and stored.
- Why is signal processing crucial for biomedical data?
- Biomedical signals often contain noise and artifacts; signal processing removes interference, enhances key features and extracts diagnostic information from ECG, EEG and other recordings.
- What are typical design challenges for implantable electronics?
- Implantable devices must be biocompatible, very low power, highly reliable, physically small and able to communicate securely through body tissues over long lifetimes.
- How do wearable health monitors use biomedical electronics?
- Wearables integrate low-power sensors, microcontrollers and wireless modules into comfortable form factors so they can continuously measure parameters such as heart rate, motion or oxygen saturation.
- Why is regulatory compliance so important in biomedical electronics?
- Regulations ensure that medical devices perform as intended, avoid harmful failures and protect patient data, making compliance essential for market approval and clinical use.
- How does telemedicine rely on biomedical electronics?
- Telemedicine systems use biomedical sensors and home monitoring devices to collect patient data remotely, transmit it securely and support clinical decisions without requiring in-person visits.
- What role do microcontrollers play in biomedical instruments?
- Microcontrollers coordinate sensor readings, run signal-processing algorithms, manage user interfaces and handle communication in compact, power-efficient medical devices.
- Which emerging trends are shaping the future of biomedical electronics?
- Key trends include flexible and wearable electronics, AI-assisted diagnosis, minimally invasive implants, and tighter integration of sensing, actuation and drug delivery at small scales.
- Electrical & Electronic Engineering topics:
- Electrical & Electronic Engineering – Overview
- Electronics Engineering
- Power Systems Engineering
- Renewable Energy & Energy Storage
- Communication Engineering
- Control Systems Engineering
- Signal Processing
- Instrumentation & Measurement
- Embedded Systems & Microelectronics
- Robotics & Automation in EE
- IoT & Smart Technologies
- Biomedical Electronics
- Quantum Electronics
Table of Contents
Core Components of Biomedical Electronics
Biomedical electronics is a multidisciplinary field that combines principles from electrical engineering, medicine, materials science, and computer science to develop devices that monitor, diagnose, treat, and even augment human physiological functions. This field serves as the foundation for technologies ranging from wearable health trackers to implantable life-saving devices. It requires precision engineering, deep understanding of biological systems, and rigorous safety compliance. The following expanded overview delves into the core components and their broader implications.
Electronic Engineering Foundations
- Sensors: Advanced sensors are designed to detect a wide range of physiological parameters—including heart rate, blood pressure, glucose concentration, neural spikes, muscle activity, oxygen saturation, respiratory rate, and even biochemical markers such as pH or electrolyte levels. Engineers work on miniaturization, biocompatibility, low power consumption, and high sensitivity. Novel sensor modalities include flexible, skin-mounted electrode arrays and implantable micro-electromechanical systems (MEMS), enabling continuous, real-time monitoring in both hospital and home settings.
- Actuators: Actuators convert electronic signals into therapeutic actions. Examples include electrical stimulators in pacemakers, deep brain stimulators for Parkinson’s, insulin pumps for diabetes management, and cochlear implants for hearing restoration. Engineers design these devices to deliver precise electrical, mechanical, or optical stimuli in response to sensor input or therapy protocols, while ensuring safety, reliability, and minimal invasiveness.
- Signal Processing: Biological signals are often noisy, non-stationary, and complex. Signal processing algorithms filter out artifacts, extract meaningful features, detect anomalies, and support data compression and pattern recognition. Techniques such as digital filters, wavelet transforms, adaptive algorithms, and machine learning models are used to enhance diagnostic accuracy, enable early detection of disease, and facilitate closed-loop control systems that autonomously adjust therapy in real time.
- Power Systems: Implantable and wearable devices require dependable power sources. Engineers work on miniaturized batteries for implants, high-density rechargeable batteries for wearable devices, and innovative energy-harvesting methods—such as piezoelectric generators, thermoelectric converters, and biofuel cells—that derive energy from body heat, movement, or biochemical reactions. Effective power management circuits regulate voltage and current to ensure consistent performance and patient safety.
Biomedical Integration
- Biocompatibility: Devices are designed using materials that are inert or bioactive, ensuring no adverse immune response. Engineers select coatings and structural designs that resist corrosion and biofouling and minimize inflammation. Long-term implantation demands deep understanding of tissue-device interactions, encapsulation strategies, and mechanical compatibility to maintain functionality and safety over years or decades.
- Miniaturization: Devices must be small and lightweight enough to integrate comfortably with the human body. This drives innovation in multi-layer PCB design, system-on-chip (SoC) technology, nanoscale components, and flexible electronics. Engineers also leverage precision assembly and microfabrication techniques for highly reliable implants and wearable arrays that seamlessly interface with biological tissues.
- Data Communication: Secure and efficient wireless communication is essential for transmitting physiological data in real time. Devices utilize protocols like Bluetooth Low Energy (BLE), Near Field Communication (NFC), medical-grade radio bands (e.g., MICS), and even infra-red or ultrasound links for implants. Data encryption, minimal latency, reliable connectivity, and battery-conscious design are critical—and wireless firmware updates maintain device functionality post-deployment.
Safety and Regulation
- Medical Standards: Biomedical electronics must meet stringent standards set by regulatory authorities—such as FDA or CE certification. Devices undergo rigorous preclinical and clinical testing for biocompatibility, electrical safety, electromagnetic compatibility (EMC), sterilization validation, and cybersecurity. Engineers must design and document devices according to ISO 13485, IEC 60601, ISO 14971, and relevant guidance to ensure patient safety and regulatory compliance.
- Reliability: Devices often operate in critical scenarios—such as life support, cardiac regulation, or neural stimulation—where failure could lead to serious harm. They must withstand mechanical stress, electromagnetic interference, temperature extremes, and moisture. Manufacturers perform accelerated life testing, redundancy design, real-time self-diagnostics, and fail-safe modes. Reliability engineering and fault tolerance are central to development.
These components together form the backbone of biomedical electronics, a field that steadily pushes toward greater personalization, autonomy, and integration of electronic systems with the human body. Engineers working in this domain not only need technical expertise but also cross-disciplinary collaboration with clinicians, materials scientists, and regulatory experts. To stay ahead of industry standards, research breakthroughs, and cross-border collaborations, consult reputable journals and platforms like the IEEE Xplore Digital Library, which provides comprehensive access to state-of-the-art research, standards, and conference proceedings.
Key Technologies in Biomedical Electronics
Biomedical electronics represents a cutting-edge intersection of engineering and healthcare, delivering innovative tools that enhance diagnosis, treatment, monitoring, and rehabilitation. Engineers design systems that interface seamlessly with the human body—whether implantable, wearable, or part of a clinical workflow—offering high precision, reliability, and patient comfort. Below is a comprehensive expansion of the core technologies shaping modern biomedical electronics, highlighting their mechanisms, applications, and future trajectories.
Implantable Medical Devices
- Pacemakers: Compact devices implanted in the chest to detect irregular heart rhythms and apply precise electrical pulses that maintain consistent heartbeat patterns, significantly reducing risks of arrhythmia-induced complications.
- Implantable Cardioverter Defibrillators (ICDs): Sophisticated implants continuously monitoring cardiac electrical activity and delivering high-voltage shocks or pacing interventions to prevent sudden cardiac death in high-risk patients.
- Cochlear Implants: Transform sounds into electrical impulses delivered directly to the auditory nerve, restoring auditory perception in individuals with severe hearing loss through multi-channel electrode arrays and signal processors.
- Neurostimulators: Devices targeting neurological conditions by delivering adaptive electrical stimuli to specific neural structures—used in Parkinson’s, epilepsy, chronic pain, and depression treatment protocols.
Diagnostic and Imaging Systems
- Magnetic Resonance Imaging (MRI): Generates high-resolution images of internal structures using strong magnetic fields and radio-frequency pulses, enabling non-invasive soft tissue visualization and functional brain mapping.
- Computed Tomography (CT) Scanners: Rapidly capture cross-sectional X-ray images for detailed 3D imaging—crucial for trauma assessment, cancer staging, and vascular studies.
- Electrocardiograms (ECGs): Surface electrodes record the heart’s electrical activity in real time for early detection of rhythm anomalies, ischemia, and structural abnormalities.
- Electroencephalograms (EEGs): Scalp-based sensors record brainwave patterns to diagnose epilepsy, sleep disorders, and cognitive pathologies through waveform analysis and event detection.
Wearable Health Monitors
- Devices like fitness trackers and smartwatches continuously measure key health metrics—heart rate, blood oxygen (SpO2), sleep quality, and physical activity—using optical sensors and embedded algorithms.
- Next-generation wearables are integrating single-lead ECG, cuffless blood pressure estimation, and minimally invasive glucose monitoring—bringing clinical-grade diagnostics to daily life.
Therapeutic Devices
- Dialysis Machines: Provide life-sustaining renal replacement by filtering blood through semi-permeable membranes and returning purified blood to patients with chronic kidney failure.
- Insulin Pumps: Automatically deliver finely tuned insulin doses via subcutaneous infusion protocols, improving glycemic control for diabetes patients through closed-loop “artificial pancreas” systems.
- Radiation Therapy Equipment: Uses precise, high-energy beams (photon or particle) to selectively target malignant cells while minimizing exposure to healthy tissues.
Biosensors and Lab-on-a-Chip Technologies
- Biosensors: Detect specific biomarkers—such as glucose, lactate, or disease-specific proteins—using electrochemical, optical, or semiconductor-based transduction. Many are integrated into portable or wearable platforms.
- Lab-on-a-Chip: Microfluidic devices that miniaturize laboratory functions like fluid handling, separation, and molecular detection onto a chip—enabling fast, point-of-care diagnostics for conditions like infectious diseases.
Prosthetics and Exoskeletons
- Bionic Limbs: Advanced prosthetics equipped with electronic sensors and actuators that interpret residual muscle signals or nerve impulses to enable intuitive motion.
- Exoskeletons: Wearable robotic suits that augment mobility for individuals with paralysis or musculoskeletal disorders, or enhance human strength in rehabilitation and industrial settings.
Telemedicine and Remote Monitoring
- Wireless-enabled devices transmit physiological data to healthcare professionals, enabling remote triage, chronic disease monitoring, and virtual consultations.
- Examples: Smart connected ECG patches, smart inhalers for respiratory conditions, continuous glucose monitors, and Wi‑Fi or cellular-capable blood pressure cuffs.
Artificial Organs and Tissue Engineering
- Artificial Hearts: Either temporary ventricular assist devices (VADs) or permanent implantable mechanical hearts that maintain circulation in end-stage cardiac patients awaiting transplantation.
- Bioelectronic Devices: Electrodes and microstimulators interfaced with living tissues—such as retinal implants that restore partial vision, or hybrid bio-hybrid systems that combine electronics with cell-based scaffolds.
Together, these technologies illustrate the breadth of biomedical electronics—from powerful in-hospital imaging systems to discreet wearable monitors and sophisticated implantables. Engineers and clinicians collaborate to push the frontiers of diagnostics, therapy, and personalized medicine. To explore the latest research, design frameworks, and standards in biomedical electronics, visit the IEEE Xplore Digital Library, a premier source for technical literature and emerging innovation.
Applications of Biomedical Electronics
Biomedical electronics has transformed modern healthcare by introducing advanced diagnostic, therapeutic, and monitoring technologies that significantly improve patient outcomes. This multidisciplinary field combines electronics, signal processing, sensor development, and biomedical insights to create devices tailored for various medical specialties. The following exploration highlights key applications across the healthcare continuum:
- Cardiology
- Devices like pacemakers, defibrillators, and ECG machines are critical for diagnosing and managing heart conditions.
Pacemakers continuously monitor heart rhythms and deliver electrical pulses to correct arrhythmias. Implantable cardioverter defibrillators (ICDs) recognize dangerous heart rhythms and deliver life-saving shocks. Surface and implantable ECG systems provide real-time cardiac monitoring in hospitals, ambulances, and even smartwatches. These technologies reduce mortality, enable early intervention, and provide long-term heart health management in outpatient settings.
- Neurology
- Neurostimulators treat conditions like epilepsy, chronic pain, and movement disorders.
- EEG and brain-computer interfaces (BCIs) facilitate understanding of brain function and development of assistive technologies.
Neurostimulators deliver targeted electrical pulses to neural pathways to manage Parkinson’s disease, depression, and refractory pain. EEG systems monitor brain activity in diagnostic and emergency contexts, aiding in seizure detection and sleep studies. Brain‑computer interfaces interpret neural signals to control prosthetic limbs, communicate with locked-in patients, or operate smart environment interfaces.
- Diabetes Management
- Continuous glucose monitors (CGMs) and insulin pumps help patients manage blood sugar levels efficiently.
CGMs measure interstitial glucose every few minutes and relay data to smartphones or insulin pump systems. Coupled with AI-driven calculators, insulin delivery systems can automatically adjust dosing. Together, these devices form an artificial pancreas, enabling tighter glucose control, fewer hypoglycemic events, and greater patient quality of life.
- Rehabilitation Medicine
- Prosthetics and robotic exoskeletons support physical therapy and recovery for patients with disabilities or injuries.
Bionic limbs equipped with electronics and sensors interpret muscle or neural signals for intuitive movement. Robotic exoskeletons support gait training for spinal injury or stroke survivors, enabling mobility and strength recovery. These wearable systems often include real-time feedback for therapists and patients.
- Critical Care
- Ventilators, dialysis machines, and patient monitors play essential roles in intensive care units (ICUs).
Ventilators electronically control airflow and oxygen delivery in respiratory failure cases. Dialysis machines cleanse blood for patients with kidney failure. Multi-parameter monitors track vital signs such as ECG, SpO₂, blood pressure, and temperature. Alarm systems integrated into these devices aid ICU staff in early detection of clinical deterioration.
- Cancer Treatment
- Imaging systems like PET and CT scanners aid in early detection, while radiation therapy machines deliver targeted treatments.
CT, MRI, and PET systems produce detailed images to characterize tumor biology and guide diagnostic and treatment decisions. Radiation oncology suites integrate linear accelerators with imaging systems for precise tumor targeting, minimizing damage to healthy tissues. Image-guided radiation systems now offer real-time tumor tracking and adaptive treatment planning.
- Public Health and Wellness
- Wearable health devices promote preventive care by tracking fitness and health metrics.
Fitness trackers, smartwatches, and connected health monitors collect data on heart rate, activity, sleep, and stress. These devices encourage healthy behavior changes through reminders and activity goals. By aggregating data across populations, they also provide valuable insights into public health trends, enabling proactive interventions.
- Surgical Applications
- Robotic surgery systems like the da Vinci Surgical System enhance precision and reduce invasiveness.
- Electrosurgical units (ESUs) allow precise cutting and coagulation during procedures.
Robotic platforms translate surgeon movements into high-precision actions, enabling minimally invasive procedures with smaller incisions and faster recoveries. Electrosurgical units deliver controlled electrical energy for tissue incision or coagulation, improving safety and outcomes. Surgical navigation systems use embedded electronics to guide instruments based on real-time imaging.
These applications demonstrate how biomedical electronics has revolutionized patient care—from hospital-critical interventions to preventive health in everyday life. Engineers in this field combine innovation with rigorous safety standards and clinical collaboration.
For further insight into emerging biomedical technologies and standards, refer to authoritative sources like the IEEE Xplore Digital Library.
Challenges in Biomedical Electronics
Biomedical electronics stands at the forefront of merging technology with human health, yet it also faces a range of unique and demanding challenges. Engineers must navigate complexities that span from the fundamental behavior of human biology to societal considerations such as affordability and data privacy. Below is a detailed expansion on the key obstacles encountered in this rapidly advancing field:
Complexity of Biological Systems
- Understanding and interacting with the human body’s intricate processes require advanced research and innovation.
Biological systems are inherently complex—dynamic, nonlinear, and variable across individuals. Capturing these systems accurately requires multi-scale modeling, from cellular interactions to organ-level functionality. Engineers work with clinicians and biologists to develop devices that can adapt to individual physiology, account for changing conditions (like inflammation, growth, or disease progression), and operate reliably within unpredictable environments.
Moreover, interfacing electronics with living tissues demands sophisticated signal transduction: converting ionic or chemical signals into electrical data. Signal noise, interference from muscle or tissue movements, and changes in tissue properties over time can degrade performance. Continuous refinement of sensors, signal filtering techniques, adaptive algorithms, and machine learning models is necessary to interpret physiological data accurately and support personalized therapies.
Power and Energy Limitations
- Many biomedical devices, especially implants, are constrained by battery life and energy requirements.
Implantable devices like pacemakers, neurostimulators, and insulin pumps require long-term, dependable power sources. Frequent battery replacements pose surgical risks, burden patients, and increase healthcare costs. Biomedical engineers are exploring avenues such as ultra-low-power electronics, wireless energy transfer, rechargeable batteries, and energy harvesting from body heat, motion, or glucose metabolism.
On the wearable front, devices must balance robustness with portability and comfort. Engineers integrate efficient power-management circuits, duty-cycling schemes, and energy-aware communication protocols. Striking this balance is critical to produce devices that patients can trust for continuous monitoring without frequent recharging or failure.
Biocompatibility and Safety
- Devices must avoid triggering immune responses or causing harm to tissues, requiring extensive testing and material innovation.
A biomedical electronic device must coexist peacefully with tissue over long periods. Immune reactions, scar tissue formation, corrosion, or mechanical degradation can impair device function or cause harm. Engineers must select materials that resist corrosion, minimize biofouling, and match tissue properties in flexibility and mechanics.
Thorough preclinical testing—including in vitro (cell culture) and in vivo (animal) trials—is essential. Regulatory paths demand decades of safety data, long-term studies, and documentation of device lifecycle, wear, and interaction with the human body. These rigorous processes, while critical, are time-consuming and costly.
Data Security and Privacy
- With increasing connectivity, ensuring the confidentiality of sensitive medical data is critical.
Modern biomedical electronics often include wireless links, cloud storage, and remote control capabilities. This introduces vulnerabilities to unauthorized access, tampering, or data breaches. Engineers must embed robust encryption, secure firmware updates, authentication protocols, and tamper-resistant hardware. Compliance with healthcare data frameworks like HIPAA or GDPR is essential.
Device failure or malicious control could have serious—and even life-threatening—consequences. Therefore, cybersecurity must be integrated into every device layer, from physical interface to communications and software.
High Development Costs
- Research, development, and regulatory approval processes for biomedical devices are expensive and time-consuming.
Developing a device from concept to clinic often spans 5–10 years and costs tens to hundreds of millions of dollars. Costs include R&D, prototyping, bench testing, animal studies, human clinical trials, manufacturing, and navigating complex regulatory reviews by bodies like the FDA or EMA.
Many engineering teams struggle to secure funding and partnerships to sustain long development cycles. Commercial viability may hinge on market size, reimbursement approvals, and healthcare infrastructure—factors that vary widely by geographical region.
Innovative pathways such as academic-industry collaborations, public–private partnerships, modular testing protocols, and regulatory fast tracks for life-saving devices aim to reduce these burdens.
Access and Affordability
- Advanced biomedical devices can be prohibitively expensive, limiting access for patients in low-income regions.
Even after overcoming technical and regulatory hurdles, these devices may still be financially out of reach for many. Developing countries and under-resourced health systems struggle to provide high-tech solutions, exacerbating global health inequities.
Engineers and nonprofits are exploring innovations in frugal engineering, lower-cost components, and open-source medical devices. These efforts aim to produce affordable and effective alternatives without compromising safety and quality.
Tackling these layered challenges requires multidisciplinary teams, international cooperation, and incentives for accessible and sustainable innovation. For engineers and researchers working at this intersection, platforms like the IEEE Xplore Digital Library remain essential repositories of knowledge and best practices.
Future Trends in Biomedical Electronics
The field of biomedical electronics stands at the forefront of healthcare transformation. Emerging technologies are rapidly converging to create smarter, smaller, and more personalized medical systems. The following expanded overview explores seven key trends that are shaping the future of this vital discipline, highlighting their potential to revolutionize diagnostics, treatment, and patient care.
AI and Machine Learning Integration
- AI algorithms enhance diagnostics, predict disease progression, and optimize treatment plans using data from biomedical devices.
Artificial intelligence is being integrated into biomedical systems to analyze vast streams of data—such as ECG, EEG, glucose trends, and imaging scans—in real time. Machine learning models can detect subtle anomalies before symptoms manifest, forecast trends like glucose fluctuations in diabetics, and suggest personalized treatment protocols. These systems also assist clinicians by automating routine analysis and flagging high-risk conditions, leading to earlier interventions and improved outcomes.
Wearable and Implantable Innovations
- New materials and miniaturization techniques enable more comfortable and effective devices for continuous health monitoring.
Biomedical engineers are developing ultra-thin, flexible electronics that conform to the skin or body, enhancing comfort and reliability over extended wear. Innovations in biocompatible polymers and stretchable circuits support long-term monitoring of vital signs such as heart rhythm, muscle activity, and biochemical markers. Implantable sensors and stimulators are becoming more discreet, with lifespans of years without needing surgical battery replacements.
Wireless and Energy-Harvesting Technologies
- Wireless power transfer and energy harvesting from body movements or heat will eliminate the need for frequent battery replacements.
Next-generation implants and wearables are being designed to draw power from the user’s own body—via thermoelectric generators, piezoelectric materials, or radio-frequency energy. Together with wireless charging, these systems reduce the risks and inconvenience of frequent device replacements. They form the foundation for true “set-and-forget” implantable health solutions that are safer, smaller, and more patient-friendly.
Personalized Medicine
- Biomedical devices tailored to individual patient needs are being developed, driven by advancements in genomics and bioinformatics.
The rise of genomic medicine and wearable monitoring facilitates highly customized therapies. For example, insulin delivery systems can adjust to diet, activity, and genetic markers; implanted neuromodulators can respond to the user’s neural patterns; and wearable devices can adjust therapy for conditions like hypertension or sleep apnea. This shift from population-based to patient-specific medicine enhances efficacy and minimizes side effects.
3D Printing and Biofabrication
- 3D-printed implants and prosthetics offer customizable and cost-effective solutions for patients.
3D printing technologies enable the on-demand creation of patient-specific implants—such as bone plates, joint replacements, and dental prosthetics—tailored to an individual’s anatomy. Biofabrication is also advancing tissue engineering by combining living cells with scaffold structures for organ repair or replacement. These technologies promise shorter lead times, fewer complications, and more personalized surgical outcomes.
Integration with Internet of Medical Things (IoMT)
- Connected devices facilitate real-time data sharing and coordination between patients and healthcare providers.
The IoMT ecosystem connects sensors, monitors, imaging devices, and hospital systems to create a unified data network. Patients wearing glucose trackers, heart monitors, or respiratory sensors can seamlessly transmit data to caregivers—triggering alerts, virtual consultations, or medication adjustments. This connected environment supports remote and continuous care, reducing hospital visits and improving chronic disease management.
Quantum Technologies
- Quantum sensors and imaging systems promise breakthroughs in sensitivity and resolution for diagnostics.
Quantum-enabled devices—such as magnetometers based on nitrogen-vacancy centers in diamonds, or ultra-sensitive stable clocks—are emerging in medical applications. They offer exceptional precision in tracking neural signals, cardiac magnetic activity, and biochemical reactions. Researchers are also exploring quantum-enhanced imaging techniques to improve resolution in MRI and nuclear medicine.
Collectively, these trends illustrate how biomedical electronics is becoming more intelligent, integrated, and patient-centered. Engineers and clinicians work together to bring these innovative technologies into everyday healthcare. To learn about the latest breakthroughs and standards in this rapidly evolving field, visit the IEEE Xplore Digital Library, an authoritative source for peer-reviewed research and industry developments.
Societal and Economic Impact of Biomedical Electronics
Improved Quality of Life
- Biomedical electronics enable early diagnosis, effective treatment, and better management of chronic diseases, significantly enhancing patient outcomes.
From wearable monitors that track heart rate and glucose levels to implantable devices like pacemakers and neurostimulators, biomedical electronics empower patients to manage their health conditions proactively. Early detection through non-invasive diagnostic tools significantly increases the chances of successful treatment. These technologies also reduce the frequency and severity of hospital admissions, allowing patients to live fuller, more independent lives. The psychological benefit of constant monitoring also contributes to peace of mind, improving mental well-being alongside physical health.
Economic Growth
- The biomedical device industry drives innovation, creating jobs and contributing to global economic development.
The biomedical electronics sector represents a dynamic and rapidly growing industry with significant contributions to national and global economies. Investment in research and development fosters innovation, spawning startups and multinational enterprises alike. These ventures create high-skill jobs in engineering, software development, and manufacturing. Additionally, the global demand for advanced medical technologies fuels export revenues and stimulates supporting sectors such as logistics, healthcare services, and insurance. According to the Statista Medical Technology Market Report, the global market for medical technologies is projected to surpass $800 billion by 2030, underscoring its substantial economic role.
Increased Access to Healthcare
- Advances in telemedicine and portable devices bring healthcare services to remote and underserved areas.
Biomedical electronics have revolutionized the accessibility of healthcare, particularly for populations in rural or resource-limited environments. Portable diagnostic devices, wireless biosensors, and smartphone-based monitoring tools bridge the gap between patients and clinicians, enabling remote consultations and continuous care. Mobile health (mHealth) platforms empower community health workers to screen and track conditions on-site, reducing reliance on centralized facilities. Moreover, solar-powered devices and low-energy solutions ensure these tools remain operational in areas with limited infrastructure, supporting equitable healthcare delivery worldwide.
Longevity and Preventive Care
- Biomedical devices empower individuals to monitor their health, reducing the burden on healthcare systems and promoting longer, healthier lives.
Preventive healthcare has gained significant momentum due to advances in biomedical electronics. Devices such as smartwatches, ECG patches, and blood pressure monitors encourage proactive health behavior, allowing users to track and manage risk factors before they escalate. This shift reduces the incidence of emergency treatments and hospitalizations, easing the strain on healthcare systems. Furthermore, ongoing monitoring of elderly populations and chronic disease patients supports independent living and decreases long-term care costs. Collectively, these benefits contribute to longer life expectancy, improved productivity, and reduced public health expenditures.
Why Study Biomedical Electronics
Integrating Engineering with Medical Science
Biomedical electronics focuses on applying electronics to healthcare and medical devices. Students learn to design systems that monitor, diagnose, and assist bodily functions. This bridges engineering innovation with patient care.
Medical Instrumentation and Signal Processing
Students study devices such as ECG machines, imaging systems, and infusion pumps. They learn how to acquire, filter, and interpret biological signals. This supports accurate diagnosis and real-time health monitoring.
Wearable and Implantable Technologies
Biomedical electronics includes designing compact systems like pacemakers and fitness trackers. Students learn how to create safe, reliable, and energy-efficient devices. These innovations improve patient mobility and quality of life.
Regulations and Clinical Compliance
Students explore standards like ISO 13485 and FDA regulations for medical devices. They understand the importance of validation, testing, and biocompatibility. This ensures products meet safety and ethical guidelines.
Contributing to Healthcare Advancements
Biomedical electronics is crucial to digital health, telemedicine, and personalized treatment. Students help create devices that transform diagnostics and therapy. The field offers rewarding careers at the intersection of engineering and medicine.
Biomedical Electronics: Conclusion
Biomedical electronics is a transformative field at the intersection of engineering and medicine. Its innovations continue to redefine the boundaries of healthcare, offering solutions to complex medical challenges and improving lives worldwide.
As one of the fastest-growing interdisciplinary domains, biomedical electronics bridges human biology with cutting-edge electronic technologies to produce devices and systems that revolutionize patient care, diagnostics, monitoring, and therapy. From wearable health trackers and implantable pacemakers to AI-assisted diagnostics and robotic surgery platforms, the impact of biomedical electronics permeates every corner of modern healthcare. These advancements not only improve outcomes for individual patients but also optimize the efficiency of entire health systems by enabling real-time data collection, predictive analysis, and remote care.
The field plays a critical role in addressing global health challenges such as the rise in chronic diseases, aging populations, and disparities in access to medical resources. Through the development of portable diagnostic devices and mobile health platforms, biomedical electronics has made it possible to deliver high-quality care to underserved regions and remote communities. Innovations in telemedicine, powered by interconnected biomedical systems, empower patients to receive consultations, monitoring, and even treatment from the comfort of their homes.
Moreover, biomedical electronics supports a paradigm shift toward preventive and personalized medicine. By integrating biosensors, wearable devices, and cloud-based analytics, individuals can continuously monitor vital health metrics and gain actionable insights into their well-being. This approach enhances disease prevention strategies, reduces long-term healthcare costs, and promotes proactive engagement in health management.
On the technological frontier, biomedical engineers and researchers are exploring groundbreaking advancements in areas such as flexible electronics, energy harvesting for implantable devices, biocompatible materials, and neural interfaces. These innovations promise to unlock new possibilities for restoring lost function, treating complex conditions, and enhancing the human body’s capabilities through bionic and biohybrid systems. For instance, brain-machine interfaces are opening up new avenues for patients with paralysis to control prosthetic limbs or communicate via thought-controlled devices.
The future of biomedical electronics is also closely tied to the rise of artificial intelligence, machine learning, and big data analytics. These tools enable faster diagnosis, automated treatment decisions, and large-scale population health monitoring. With responsible data governance and robust cybersecurity frameworks, they also ensure the ethical use of sensitive patient information in this digital age. Initiatives like the NIH Precision Medicine Initiative exemplify the promise of integrating biomedical electronics with AI and genomics for individualized treatment pathways.
Importantly, the field is committed to regulatory compliance, safety, and accessibility. Biomedical devices must adhere to stringent standards—such as FDA or CE approval—to guarantee safety, efficacy, and reliability, particularly in life-critical applications. As technology becomes more embedded in everyday life, manufacturers and policymakers alike are striving to ensure that innovations remain affordable, inclusive, and equitable for global populations.
In conclusion, biomedical electronics is not merely an auxiliary aspect of healthcare—it is a foundational driver of its evolution. By merging precision engineering with deep biological insight, this field continues to push the boundaries of what is possible in medicine. As new technologies emerge and integrate, biomedical electronics will remain at the forefront of a healthcare revolution, fostering a healthier, more connected, and more resilient world for generations to come.
Biomedical Electronics – Frequently Asked Questions (FAQ)
1. What does “biomedical electronics” actually cover?
Biomedical electronics covers the design of electronic circuits, sensors and systems that interact with the human body. This ranges from simple ECG amplifiers and pulse oximeters to complex medical imaging equipment, implantable pacemakers and wearable health trackers.
2. How is biomedical electronics different from general consumer electronics?
Consumer electronics focus on cost, performance and user experience, whereas biomedical electronics must also satisfy strict safety, reliability and regulatory constraints. Devices must be safe in contact with the body, robust against failure and accurate enough to support clinical decisions.
3. Why are biosensors so central to biomedical devices?
Biosensors translate chemical or biological quantities, such as blood glucose or oxygen saturation, into electrical signals. Without this translation, it would be impossible for electronic systems to “see” what is happening inside the body in real time.
4. What kinds of signals are processed in biomedical electronics?
Common signals include electrical activity from the heart (ECG), brain (EEG) and muscles (EMG), as well as optical, pressure and chemical signals from imaging or blood-chemistry measurements. Each type of signal requires specific conditioning and analysis techniques.
5. Why is low power consumption such a big issue for medical devices?
Many biomedical devices are battery-powered, either as wearables or implants. Low power consumption extends battery life, reduces the frequency of surgeries for implant replacement and allows small, comfortable designs that patients are more willing to use.
6. Are wearable health gadgets and hospital equipment based on similar principles?
Yes. Both rely on sensors, amplifiers, filters, microcontrollers and wireless communication. The main differences lie in performance requirements, data accuracy, robustness and how strictly the devices are regulated for clinical use.
7. How does biomedical electronics support telemedicine and remote care?
Home-based sensors, smart patches and wearable monitors can continuously collect data and send it to healthcare providers. This supports remote diagnosis, chronic disease management and early detection of problems without frequent clinic visits.
8. What career paths are open to students who study biomedical electronics?
Graduates can work in medical device design, hospital equipment support, regulatory compliance, health-tech startups, rehabilitation technology, imaging system development and research labs focused on new diagnostic or therapeutic technologies.
9. Do I need a strong biology background to succeed in biomedical electronics?
A basic understanding of human physiology is helpful, but the core skills are in circuits, signal processing, embedded systems and systems engineering. You can gradually build your biomedical knowledge while applying engineering tools to real healthcare problems.
10. How is AI starting to influence biomedical electronic systems?
AI algorithms are increasingly embedded in medical devices and cloud platforms to interpret complex signals, detect abnormal patterns and support clinical decision-making. This can make diagnostics faster, more consistent and more personalised for each patient.
Biomedical Electronics: Review Questions with Detailed Answers
Biomedical electronics brings together electronic circuits, sensors and embedded systems to work safely with the human body. The questions below help you revise key ideas about how medical devices sense, process and act on physiological information in real clinical and home-care settings.
-
What is biomedical electronics, and how does it differ from conventional electronics?
Answer: Biomedical electronics is the application of electronic engineering to medical devices and systems that measure, monitor or influence physiological processes. Examples include ECG machines, implantable pacemakers, infusion pumps and wearable health trackers. In contrast to general electronics, biomedical systems must meet strict requirements for patient safety, biocompatibility, electrical isolation and accuracy in handling biological signals. They are also developed under healthcare regulations and standards that govern design, testing and clinical use. -
How do biosensors and transducers enable the monitoring of physiological signals?
Answer: Biosensors and transducers convert biological or chemical quantities into electrical signals that electronic circuits can process. A biosensor typically combines a selective sensing element—such as an enzyme, antibody or ion-selective membrane—with a transducer that responds to changes in voltage, current or impedance. When the target analyte, such as glucose or oxygen, interacts with the sensing layer, the transducer produces an electrical output proportional to its concentration. This signal can then be amplified, filtered and digitised to support real-time monitoring and diagnosis. -
In what ways do electronic circuits underpin medical imaging technologies like MRI and ultrasound?
Answer: Medical imaging systems rely on electronics at every stage: generating excitation signals, receiving weak responses and reconstructing images. In MRI, power electronics drive gradient and radiofrequency coils that excite hydrogen nuclei, while low-noise amplifiers and high-speed digitizers capture the tiny signals emitted as nuclei relax. In ultrasound, piezoelectric transducers convert electrical pulses into sound waves and back into electrical echoes. Signal-conditioning circuits then amplify and filter these echoes before digital processors form cross-sectional images. Without precise timing, stable power supplies and sensitive analog front-ends, the high-resolution imaging used in modern diagnostics would not be possible. -
Why is signal processing a critical step in analysing biomedical data such as ECG or EEG recordings?
Answer: Raw biomedical signals often contain noise from muscle activity, electrode motion, mains interference and other sources that can mask clinically important features. Signal processing techniques—such as filtering, baseline wander removal, feature extraction and pattern recognition—are used to clean and interpret these recordings. For example, in ECG analysis, processing helps identify P, QRS and T waves and detect arrhythmias, while in EEG analysis it supports localisation of abnormal brain activity or seizure prediction. Effective signal processing improves diagnostic reliability and can enable automated decision support in monitoring systems. -
What are some key design considerations for implantable medical devices like pacemakers?
Answer: Implantable devices must operate safely and reliably inside the body for many years. Important design considerations include biocompatible packaging to avoid adverse immune reactions, extremely low power consumption to maximise battery life, and robust circuitry that can tolerate temperature and chemical variations in tissue. Devices like pacemakers need precise sensing of cardiac signals, dependable timing circuitry for pacing pulses and fail-safe behaviour if any component degrades. Engineers also consider secure wireless communication for device programming and follow-up, while guarding against unintended interference or unauthorised access. -
How do wearable health monitors balance user comfort, battery life and reliable data collection?
Answer: Wearable devices must be comfortable enough for long-term use while still collecting high-quality data. Designers use soft straps, flexible circuit boards and skin-friendly materials to reduce irritation and bulk. To extend battery life, they employ low-power microcontrollers, energy-efficient sensors and communication protocols such as Bluetooth Low Energy. Intelligent duty-cycling reduces measurement and transmission rates when the user is inactive, while calibration and signal-processing algorithms compensate for motion artifacts and changes in sensor placement. The result is a device that users are willing to wear daily and clinicians can trust for trend analysis. -
Why are safety and regulatory compliance paramount in biomedical electronic systems?
Answer: Biomedical devices directly affect patient health, so failures can have serious consequences. Safety standards and regulations ensure devices are electrically safe, function correctly under specified conditions and provide reliable information to clinicians. Engineers design in protective measures such as isolation barriers, current limits, alarms and redundancy to prevent hazardous situations. They also follow formal processes for risk analysis, verification, validation and clinical evaluation. Meeting regulatory requirements is essential not only for legal approval but also for maintaining trust in medical technology. -
How do telemedicine and remote patient monitoring make use of biomedical electronics?
Answer: Telemedicine relies on biomedical electronics to collect physiological data outside traditional clinical settings. Home blood-pressure cuffs, pulse oximeters, ECG patches and multi-sensor wearables record vital signs and transmit them via secure wired or wireless links to healthcare providers. Embedded electronics manage local data storage, preliminary analysis and connectivity to smartphones or home hubs. This continuous or frequent monitoring supports early detection of problems, personalised therapy adjustments and reduced need for hospital visits, especially for chronic disease management. -
What role do microcontrollers and embedded systems play in advanced diagnostic and therapeutic devices?
Answer: Microcontrollers serve as the central control units in many diagnostic and therapeutic devices. They coordinate sensor acquisition, run signal-processing algorithms, manage user interfaces and handle communication with external systems or hospital networks. Embedded firmware can implement safety checks, adapt device behaviour to patient-specific settings and enable over-the-air updates to improve performance over time. By integrating multiple functions on a single low-power chip, microcontrollers make complex biomedical functionality possible in compact and portable instruments. -
What emerging trends are likely to shape the future of biomedical electronics?
Answer: Several trends are reshaping this field, including flexible and stretchable electronics that conform to the body, smart textiles with integrated sensors, and minimally invasive or injectable devices for continuous monitoring. Advances in artificial intelligence enable more sophisticated interpretation of complex physiological data and support predictive diagnostics. At smaller scales, nanotechnology and lab-on-a-chip platforms promise highly sensitive, point-of-care tests. Together, these developments are moving biomedical electronics toward more personalised, continuous and unobtrusive healthcare.
Biomedical Electronics: Review Questions with Detailed Answers:
1. What is biomedical electronics, and how does it differ from conventional electronics?
Answer:
Biomedical electronics is the application of electronic engineering principles to the design and development of medical devices and systems that diagnose, monitor, or treat health conditions. Unlike conventional electronics, which may focus primarily on consumer or industrial uses, biomedical electronics must address stringent requirements such as patient safety, biocompatibility, and accurate physiological data handling.
Moreover, biomedical electronic systems operate in or around the human body, demanding specialized components and designs to ensure minimal harm. Engineers often consider additional regulations, sterilization protocols, and reliability standards to protect patient well-being and maintain consistent performance in sensitive healthcare environments.
2. How do biosensors and transducers play a vital role in monitoring physiological signals?
Answer:
Biosensors and transducers convert biological or chemical information (such as glucose levels or neural activity) into measurable electrical signals. They typically rely on specialized sensing elements—enzymes, antibodies, or other biochemical materials—that selectively interact with target molecules. This targeted interaction enables the device to capture accurate, real-time data on a patient’s condition.
Once the biosensor detects a specific biological marker, an embedded transducer then translates that signal into a voltage, current, or frequency output, which can be processed and displayed. This seamless chain of detection and conversion forms the backbone of modern patient monitoring, fueling diagnostic tools like continuous glucose monitors, pulse oximeters, and wearable heart-rate trackers.
3. In what ways do electronics underpin medical imaging technologies such as MRI and ultrasound?
Answer:
Medical imaging relies heavily on electronic components for signal generation, detection, and processing. For example, MRI (Magnetic Resonance Imaging) equipment uses high-powered magnets and radiofrequency pulses controlled by electronic systems to excite hydrogen nuclei in the body. Advanced processing electronics capture the tiny signals emitted as these nuclei return to their resting states, constructing detailed cross-sectional images of internal tissues.
Ultrasound machines likewise depend on piezoelectric transducers that generate high-frequency sound waves and detect their echoes. The echoes, converted into electrical signals, require sophisticated filtering and amplification to form real-time images of organs and fetuses. Without precise electronic control, these imaging modalities would be unable to achieve the clarity, resolution, and safety levels required in modern healthcare diagnostics.
4. Why is signal processing a critical step in analyzing biomedical data like ECG or EEG recordings?
Answer:
Signal processing is essential because raw biomedical signals—like electrocardiograms (ECGs) or electroencephalograms (EEGs)—often contain noise and artifacts that can obscure key diagnostic information. By applying filters, amplifiers, and algorithms to the data, engineers and clinicians can isolate relevant waveforms or patterns that indicate cardiac events, neurological activity, or other physiological states.
Beyond simply “cleaning” the signal, advanced processing techniques can identify arrhythmias, predict seizures, or even guide automated medical devices. This enhances diagnostic accuracy, fosters early intervention, and paves the way for intelligent systems that adjust treatments in real time based on precise signal metrics.
5. What are some design considerations for implantable medical devices like pacemakers?
Answer:
Implantable devices must operate reliably within the human body over extended periods, typically without direct user intervention. A critical design factor is biocompatibility—ensuring that the device’s materials do not provoke an immune response. Additionally, these devices must function with minimal power consumption to prolong battery life or make battery replacements infrequent.
Engineers also account for miniaturization, as the device must be physically compatible with the body’s anatomy. Robust wireless communication is another key aspect, allowing data transfer or device programming without invasive procedures. Finally, fail-safe mechanisms are crucial: if any part of the pacemaker malfunctions, the device should have the capacity to maintain its critical function or alert healthcare providers immediately.
6. How do wearable health monitors balance user comfort, battery efficiency, and reliable data collection?
Answer:
Wearables must be lightweight, ergonomic, and skin-friendly to encourage consistent use. Designers often prioritize sleek form factors and breathable materials for straps or patches. To maintain comfort, data-collection modules are minimized, and electronics are efficiently arranged to avoid bulky or protruding components.
Battery efficiency is managed by using low-power wireless protocols (like Bluetooth Low Energy), incorporating motion or event-based data sampling, and employing energy-saving microcontrollers. Meanwhile, accurate sensing algorithms and calibrated sensors ensure reliable data collection, enabling functionalities such as heart-rate or SpO₂ monitoring without compromising the user’s daily activities or comfort.
7. Why is safety and regulatory compliance paramount in biomedical electronics, and how do engineers uphold these standards?
Answer:
Biomedical devices interact directly with patients, so any fault—like electrical leakage, inaccurate data reporting, or mechanical failure—can pose serious health risks. Safety standards and regulatory compliance (as defined by regional health authorities) ensure these products consistently work as intended and protect users from harm.
Engineers uphold these standards by incorporating protective features such as insulation barriers, redundant circuits, and robust fault detection methods. Rigorous testing and validation procedures, including simulations and clinical trials, help confirm reliability under various conditions. Post-deployment monitoring further verifies that any unexpected issues are promptly identified and addressed.
8. How do telemedicine and remote patient monitoring leverage modern biomedical electronics for better healthcare outcomes?
Answer:
Telemedicine devices enable doctors to monitor and treat patients without requiring them to visit a clinical facility. Biomedical electronics, such as wearable sensors or home-based diagnostic tools, collect real-time data on vital signs, medication adherence, or disease progression.
These devices often send collected information via secure wireless networks to healthcare providers, who can then analyze trends and adjust treatment plans remotely. This approach improves access to care, particularly for individuals with limited mobility or those in remote regions. It also reduces healthcare costs by minimizing in-person visits and preventing hospital admissions through early intervention.
9. What role do microcontrollers and embedded systems play in advanced diagnostic devices, and why are they vital?
Answer:
Microcontrollers serve as the “brains” of many diagnostic devices, managing sensor inputs, processing data, and controlling user interfaces. They integrate CPU cores, memory, and communication peripherals on a single chip, making devices more compact and power-efficient.
Their importance lies in their flexibility and programmability: engineers can tailor algorithms or add new functionalities through software updates. This ensures rapid iteration and refinement of diagnostic capabilities, enabling more sophisticated data analysis, improved patient outcomes, and compatibility with emerging technologies such as machine learning.
10. What emerging trends may shape the future of biomedical electronics?
Answer:
Technological convergence is poised to redefine healthcare. For instance, miniaturized biosensors and flexible electronics can be integrated into smart textiles that continuously monitor vital signs. Advancements in battery technology and wireless charging may remove the need for invasive procedures to replace power sources in implantable devices.
Artificial intelligence stands to enhance predictive diagnostics, sifting through massive datasets to spot patterns undetectable by humans. Nanotechnology-based implants and personalized devices, capable of delivering drugs or monitoring specific health conditions at the cellular level, herald a new era of hyper-targeted treatments. These developments collectively point to a future in which medical electronics are increasingly autonomous, connected, and personalized, significantly boosting the quality of patient care.
