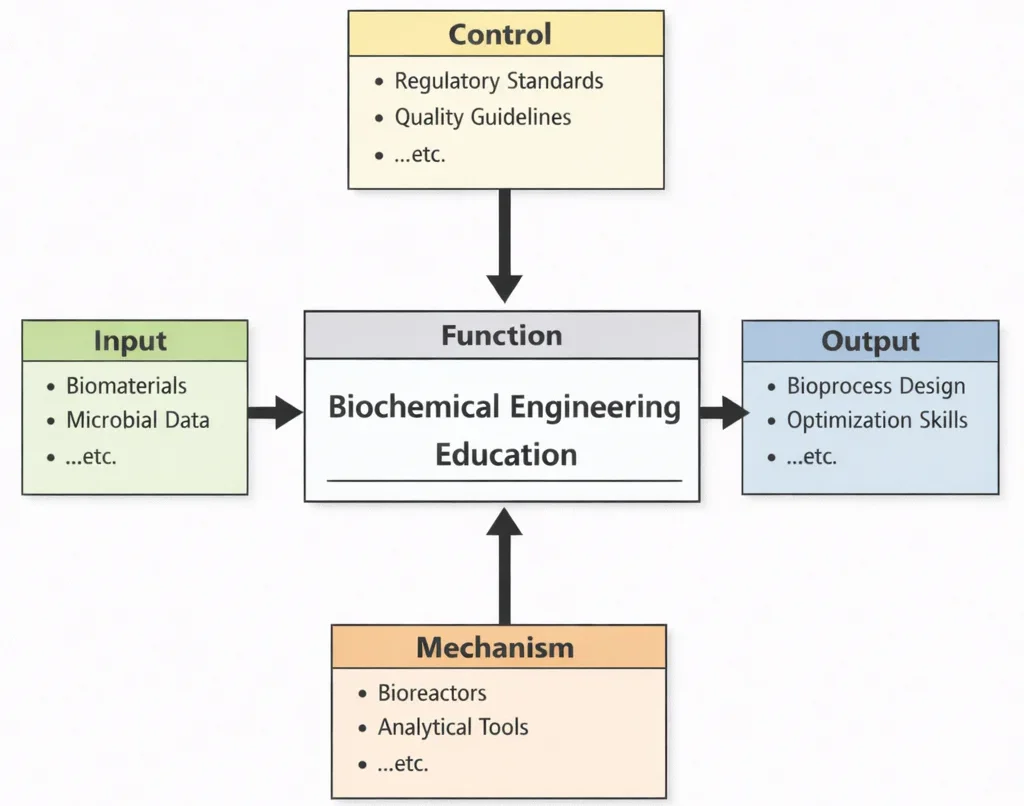
Biochemical Engineering Education, viewed through this diagram, teaches students how to translate living complexity into engineered reliability. Inputs anchor learning in real biological variability—growth behavior, reaction kinetics, mass transfer limits, and the messy signals that come from working with cells and enzymes. Controls ensure that experimentation and scale-up remain safe, ethical, and reproducible, so results can be trusted beyond the lab bench. Mechanisms—especially bioreactors and measurement tools—turn theory into practice: students learn to set conditions, track key variables, detect deviations, and refine processes based on evidence. The outputs are graduates who can design and operate bioprocesses with engineering discipline, producing consistent outcomes for applications such as fermentation products, biopharmaceuticals, enzymes, biomaterials, and other bio-based manufacturing pathways.
Biochemical Engineering bridges the gap between biology and chemical process design, enabling large-scale production of bioproducts such as enzymes, biofuels, vaccines, and antibiotics. Rooted in the foundational principles of Chemical Engineering, it expands the scope to include living systems and biochemical reactions. Through advanced understanding of Chemical Catalysis and Reaction Engineering, engineers design bioreactors that support cell cultures or microbial processes efficiently and safely.
Modern bio-based industries rely on innovative energy integration, informed by Chemical Energy Systems Engineering to optimize energy flow in fermentation and downstream purification. Materials selection for bioprocess equipment often involves insights from Chemical Materials Engineering, especially when dealing with sterilization, corrosion resistance, or biocompatibility. To ensure continuous and cost-effective operations, engineers apply the methods of Chemical Process Engineering to biochemical environments.
Simulation tools and digital twins have transformed process design, thanks to the tools taught in Computational Chemical Engineering. Applications extend into Food and Beverage Engineering, where microbial and enzymatic processes are used to create fermented foods and extend shelf life. In cutting-edge research, the nanoscale behavior of enzymes and proteins is explored in Nanotechnology in Chemical Engineering.
Plastics and bio-polymers used for bioreactors, culture vessels, and packaging require a solid grasp of Polymer and Plastics Engineering. Biochemical facilities must also interface with infrastructure designed by Civil Engineering professionals to ensure utility access, safety, and regulatory compliance. Proper site execution and project timelines draw on principles from Construction Management.
Environmental safeguards for chemical and biological spills are a core concern addressed in Earthquake and Disaster Engineering, while storage design and terrain analysis relate to Geotechnical Engineering. Building codes and load-bearing requirements are set by Structural Engineering. Transportation of bio-products or hazardous materials is safely planned using methods from Transportation Engineering and distribution layouts from Urban and Regional Planning.
Waste management and water treatment in fermentation plants are tackled through knowledge from Water Resources Engineering. Automation in fermentation control and downstream processing depends on innovations from Electrical and Electronic Engineering, including bio-compatible sensors from Biomedical Electronics. For real-time monitoring and alarms, data transmission systems from Communication Engineering are integrated into bioprocess networks.
Efficient bioreactor control requires feedback loops and regulatory algorithms provided by Control Systems Engineering. Signal conversion and amplification tools are developed using Electronics Engineering, and embedded microcontrollers from Embedded Systems and Microelectronics ensure autonomous control of batch and continuous processes. The backbone of measurement accuracy and compliance with GMP standards lies in Instrumentation and Measurement, completing the technological framework that supports the dynamic and interdisciplinary field of Biochemical Engineering.

This illustration portrays biochemical engineering as a meeting point between living systems and industrial process design. A researcher monitors experiments with a tablet while large illuminated DNA structures symbolize genetic and cellular foundations—how microbes or cells can be guided to make useful compounds. Around them are bioreactor-like chambers, test tubes, and flasks containing vivid green cultures, representing fermentation, cell cultivation, and bioprocess development. The floating graphs and molecular diagrams suggest process control, measurement, and optimisation: tracking growth, yield, purity, temperature, pH, and nutrient levels to keep biological production stable and efficient. Together, the elements communicate the core idea of biochemical engineering: turning biological reactions into reliable, scalable manufacturing systems for pharmaceuticals, enzymes, vaccines, biofuels, and environmentally friendlier chemicals.
Biochemical Engineering – Invisible FAQ
- How is biochemical engineering different from biotechnology?
- Biotechnology focuses on using living systems or biological molecules to create useful products, often at laboratory or pilot scale. Biochemical engineering takes these biological ideas and designs full-scale processes, equipment and control strategies so that production can run safely, efficiently and consistently in industrial settings.
- What scientific foundations are essential for studying biochemical engineering?
- Students typically need a strong base in biology (microbiology, cell biology, biochemistry), chemistry, thermodynamics, transport phenomena, reaction engineering and statistics. Increasingly, skills in bioinformatics, programming and data analytics are also valuable for modelling and optimising bioprocesses.
- What types of products are manufactured using biochemical engineering principles?
- Biochemical engineering underpins the production of therapeutic proteins, vaccines, antibiotics, amino acids, organic acids, biofuels, bioplastics, enzymes, fermented foods and many other high-value bioproducts. It also supports emerging areas such as cultured meat and cell-based therapies.
- Why are bioreactors central to biochemical engineering practice?
- Bioreactors provide controlled environments where cells, enzymes or tissues can grow and carry out specific biochemical transformations. Engineers must manage mixing, oxygen transfer, nutrient delivery, pH and temperature so that biological systems remain healthy and productive at scale.
- What makes scaling up bioprocesses more challenging than scaling up purely chemical processes?
- Living cells are sensitive to shear, temperature, pH, nutrient gradients and toxic by-products. When moving from small flasks to large reactors, maintaining uniform conditions and adequate oxygen and nutrient transfer becomes difficult. Scale-up strategies must protect biological activity while still achieving high productivity and product quality.
- How do metabolic engineering and synthetic biology extend the capabilities of biochemical engineering?
- Metabolic engineering and synthetic biology allow engineers to redesign cellular pathways and create new genetic circuits. This enables microbes and cell cultures to produce higher titres of existing products, to use cheaper feedstocks, or to synthesise entirely new molecules that were previously inaccessible through biology.
- What role do bioseparation and purification technologies play in bioprocessing?
- Because bioproducts are often fragile and present at low concentrations in complex mixtures, separation and purification steps dominate the cost and complexity of many bioprocesses. Techniques such as filtration, centrifugation, chromatography and crystallisation are engineered to maximise yield and purity while protecting biological activity.
- How is data analytics used in modern biochemical engineering?
- Data analytics and machine learning support soft sensors, real-time optimisation, fault detection and advanced control in bioreactors and purification trains. They also help interpret omics data, guide strain improvement programmes and accelerate process development by linking high-throughput experiments with predictive models.
- What ethical and regulatory issues are closely associated with biochemical engineering?
- Biochemical engineering intersects with regulations on genetically modified organisms, biopharmaceutical manufacturing, biosafety and environmental release. Ethical questions arise around gene editing, access to biologic therapies, sustainability of feedstocks and responsible innovation in areas such as cultured meat and human cell engineering.
- Which career paths are open to graduates specialising in biochemical engineering?
- Graduates can work in biopharmaceutical manufacturing, vaccine production, food and beverage fermentation, industrial biotechnology, biofuels, environmental bioremediation, regulatory affairs, process development, technical consulting and research roles in both academia and industry.
- Chemical Engineering topics:
- Chemical Engineering – Overview
- Chemical Process Engineering
- Chemical Catalysis & Reaction Engineering
- Chemical Materials Engineering
- Chemical Energy Systems Engineering
- Polymer & Plastics Engineering
- Nanotechnology in Chemical Engineering
- Biochemical Engineering
- Food & Beverage Engineering
- Computational Chemical Engineering
Table of Contents
Core Concepts in Biochemical Engineering
Bioprocess Development
Bioprocess development encompasses the design, optimization, and implementation of biological systems for the production of valuable products such as pharmaceuticals, biofuels, and food additives. This multidisciplinary approach integrates biology, chemistry, and engineering principles to efficiently convert raw materials into desired end products.
Stages of Bioprocessing
- Upstream Processing:
- This phase involves selecting appropriate microorganisms or cell lines, preparing nutrient media, and cultivating cells under optimal growth conditions.
- Key factors include nutrient composition, temperature, pH, and oxygenation levels.
- Common technologies include bioreactors, cell culture systems, and nutrient delivery methods.
- Downstream Processing:
- This stage focuses on harvesting the product from the bioreactor broth, purification, and final product formulation.
- Processes include centrifugation, filtration, chromatography, crystallization, and drying techniques.
- Efficient downstream processing ensures high purity, product stability, and compliance with regulatory standards.
Industrial Applications
- Production of vaccines, antibiotics, therapeutic proteins, and biofuels.
- Development of bio-based products such as biodegradable plastics and specialty chemicals.
Fermentation Technology
Fermentation technology employs microorganisms to convert raw materials into desired biochemical products through controlled metabolic processes. It’s central to biochemical engineering and widely applied across various industries, from healthcare to food production.
Types of Fermentation
- Batch Fermentation:
- Cells grow in a closed system with no additions during the process, suitable for products that require controlled conditions for optimal yield.
- Continuous Fermentation:
- A steady-state system where nutrients are continually supplied and products removed, useful for consistent, large-scale production.
- Fed-Batch Fermentation:
- A hybrid method involving periodic feeding of nutrients to prolong the productive phase and enhance yields.
Key Products
- Antibiotics (penicillin), probiotics, bioethanol, enzymes, vitamins, and organic acids.
- Industrial-scale production of fermented foods and beverages.
Bioreactor Design and Operation
Bioreactors are specialized vessels designed to provide optimal conditions for biological reactions and cell growth. Effective bioreactor design and operation are critical to maximize productivity, ensure reproducibility, and maintain product quality.
Types of Bioreactors
- Stirred-Tank Bioreactors (STR): Commonly used for microbial and cell culture processes, providing uniform mixing and oxygen transfer.
- Airlift Bioreactors: Suitable for shear-sensitive cultures, relying on air bubbles for mixing.
- Packed-Bed Bioreactors: Contain immobilized cells or enzymes, ideal for continuous and efficient conversion processes.
Operational Considerations
- Control of temperature, pH, dissolved oxygen, agitation rate, and nutrient supply.
- Implementation of automated control systems for process optimization.
Downstream Processing
Downstream processing involves isolating, purifying, and formulating biochemical products obtained from fermentation or cell culture processes. Efficient downstream processing is essential to achieving high purity and regulatory compliance for pharmaceuticals and biologics.
Common Techniques
- Filtration and Centrifugation: Separating cells and debris from product-containing fluids.
- Chromatography: High-resolution purification based on differences in molecular size, charge, affinity, or hydrophobicity.
- Drying and Formulation: Stabilizing the final product to ensure long-term storage and effective administration.
Enzyme Engineering and Biocatalysis
Enzyme engineering involves modifying enzymes to improve their catalytic efficiency, stability, and selectivity for industrial processes. Biocatalysis harnesses enzyme capabilities to facilitate environmentally friendly chemical reactions, aligning with principles of green chemistry.
Industrial Uses
- Production of bio-based chemicals, pharmaceuticals, food processing, and biodegradable materials.
- Improvement of reaction conditions, reducing energy consumption and environmental impact.
Metabolic and Genetic Engineering
Metabolic engineering manipulates cellular metabolic pathways to optimize the production of valuable compounds, while genetic engineering provides the tools to insert, delete, or modify genes within organisms.
Techniques and Applications
- CRISPR-Cas9: Advanced gene-editing technology allowing precise genetic modifications.
- Synthetic Biology: Constructing artificial biological pathways and systems for customized biochemical production.
- Production of biofuels, therapeutic proteins, bioplastics, and industrial enzymes.
Emerging Technologies in Biochemical Engineering
New methodologies and technologies continually enhance biochemical engineering capabilities, enabling efficient, sustainable, and cost-effective production methods.
Innovative Approaches
- Single-use bioreactors for rapid scale-up and reduced contamination risks.
- Microfluidic bioreactors enabling precise control over cellular environments.
- Integration of artificial intelligence (AI) and machine learning to optimize bioprocess conditions and predict outcomes.
Regulatory and Ethical Considerations
Biochemical engineering operates under stringent regulatory frameworks, particularly for pharmaceuticals and genetically modified organisms. Ethical considerations include biosafety, environmental impact, and public acceptance.
Key Regulatory Bodies and Frameworks
- U.S. Food and Drug Administration (FDA) oversees approval and compliance of bioproducts.
- European Medicines Agency (EMA) regulates biopharmaceutical products within Europe.
By mastering these core concepts, biochemical engineers continue to drive innovations that advance healthcare, sustainability, and industrial biotechnology.
Key Applications of Biochemical Engineering
Pharmaceutical Production
- Biopharmaceuticals:
- Production of therapeutic proteins, monoclonal antibodies, and vaccines.
- Microbial fermentation for antibiotics (e.g., penicillin, erythromycin).
- Cell and Gene Therapies:
- Engineered cells for cancer therapy (CAR T-cell therapy).
- Gene editing for treating genetic disorders.
Biofuels and Renewable Energy
- Bioethanol Production:
- Fermentation of sugars from crops like corn and sugarcane.
- Biodiesel:
- Enzymatic transesterification of vegetable oils.
- Algal Biofuels:
- Cultivating algae for lipid extraction and conversion into fuel.
- Biogas:
- Anaerobic digestion of organic waste to produce methane-rich gas.
Industrial Enzymes and Biocatalysts
- Applications:
- Enzymes in detergents, textiles, and food processing.
- Biocatalysts for green chemical synthesis.
Food and Beverage Industry
- Applications:
- Fermentation processes for producing yogurt, cheese, beer, and wine.
- Enzymes for flavor enhancement and texture improvement.
Waste Treatment and Environmental Biotechnology
- Bioremediation:
- Microorganisms to degrade environmental pollutants.
- Wastewater Treatment:
- Biological treatment processes using activated sludge and biofilms.
- Bio-based Plastics:
- Production of biodegradable plastics like polylactic acid (PLA).
Agriculture and Biofertilizers
- Applications:
- Production of biofertilizers and biopesticides.
- Genetically engineered crops for improved yield and resistance.
For further reading, see Nature: Biochemical Engineering and ScienceDirect: Biochemical Engineering Journal.
Emerging Technologies in Biochemical Engineering
1. Synthetic Biology: Engineering Life’s Building Blocks
Definition: Synthetic biology involves designing and constructing novel biological systems or reprogramming existing organisms to perform custom functions—essentially engineering life.
- Microbial Factories: Genetically engineered microbes (such as E. coli or yeast) are programmed to churn out high-value products like pharmaceuticals, industrial enzymes, or specialty chemicals with high yield and purity.
- Metabolic Pathway Engineering: Scientists assemble new metabolic circuits in microbes to transform cheap feedstocks (e.g., sugar, glycerol) into complex compounds—bio-based plastics, fragrances, or pharmaceuticals.
- Biocontainment Strategies: Advanced designs include “kill switches” or dependency systems to prevent engineered organisms from surviving outside controlled environments, reducing biosafety risks.
2. CRISPR & Gene Editing: Precision Engineering
Definition: CRISPR-Cas and related gene-editing tools allow unprecedented precision in modifying DNA sequences, enabling targeted modifications in cells and organisms.
- Enhanced Microbial Production: CRISPR is used to delete or enhance genes in microbes to optimize biochemical pathways—for example, improving yields of biofuels or therapeutic precursors.
- Cell Line Development: Industrial cell lines (CHO cells, yeast) are engineered to produce more stable, higher-quality biologics such as monoclonal antibodies or vaccines.
- Therapeutic Applications: In human cells, CRISPR is being investigated for treating genetic disorders—through ex vivo editing of patient cells to restore function or deliver therapeutic products.
- Regulatory & Ethical Considerations: Precision editing brings ethical debates about germline modifications, off-target effects, and long-term safety, requiring integrated governance frameworks.
3. Microbial Fuel Cells (MFCs): Waste to Watts
Definition: MFCs harness bacteria’s metabolic processes to degrade organic matter and simultaneously generate electric current.
- Renewable Energy from Waste: These systems offer a sustainable way to treat wastewater (municipal or agricultural) while producing renewable electricity.
- Portable Power Applications: Emerging low-power devices (sensors, environmental monitors) can be powered by MFCs in remote or off-grid settings.
- Enhancing Performance: Research focuses on improving electrode materials, optimizing microbial consortia, and scaling up to enhance power density and economic feasibility.
4. Algal Biotechnology: Harnessing Photosynthesis
Definition: Using algae as versatile biofactories for fuels, pharmaceuticals, and biomaterials – tapping into their rapid photosynthetic growth.
- Biofuel Production: Algae can be cultivated to produce lipids, which are then converted into biodiesel or other renewable fuels more sustainably than conventional crops.
- High-Value Bioproducts: Certain algae produce pigments, omega-3 fatty acids, antioxidants, and pharmaceuticals that have commercial and health benefits.
- Carbon Capture Integration: Algal cultivation systems can serve as bio-sinks, sequestering CO2 from industrial emissions or the atmosphere and converting it into biomass.
5. Continuous Bioprocessing: Next‑Gen Production Systems
Definition: Continuous bioprocessing keeps production lines running uninterrupted, removing batch-based start-stop inefficiencies.
- Higher Productivity: Continuous systems can operate 24/7 in steady state, increasing output volume while maintaining consistent product quality.
- Lower Costs & Footprint: Such systems reduce downtime, minimize energy expenditures, and diminish cleaning cycles, lowering both capital and operating costs.
- Real-Time Monitoring: Integration with sensors and automation facilitates real-time quality control (Quality by Design), allowing immediate adjustments and reducing batch failures.
- Adoption in Pharma: The biotech and vaccine sectors are increasingly adopting continuous manufacturing for critical drugs, as supported by regulatory guidelines such as the FDA’s emphasis on these systems.
Outlook and Integration
- Synergies Between Technologies: Techniques like CRISPR, synthetic biology, and continuous processing are transforming bioproduction—engineered organisms in continuous systems promise full, efficient pipelines from gene to product.
- Scale and Sustainability: These emerging technologies aim to shift biochemical engineering toward a future of green manufacturing, closed-loop conversion systems, and sustainable bioproduction integrated with environmental remediation.
- Cross‑Sector Applications: Whether it’s producing next-gen medicines, clean fuels, or carbon-neutral chemicals, these tools are converging—transforming agriculture, healthcare, energy, and materials.
For further reading, visit the MIT Synthetic Biology platform and the official site of CRISPR Therapeutics to explore real-world applications.
Challenges in Biochemical Engineering
1. Process Scale-Up
Scaling a bioprocess from the controlled environment of a laboratory to a full industrial production facility involves complex challenges. Small-scale success does not guarantee efficiency or yield at scale. Bioreactor engineering must accommodate factors like oxygen transfer rates, mixing efficiency, temperature control, and pH stability. Engineers need to redesign equipment and processes to maintain productivity while preventing unwanted side reactions and ensuring consistency under industrial conditions.
2. Contamination Control
Maintaining sterility is critical, as microbial contamination can derail entire production batches. Engineering robust sterilization protocols, designing bioreactors that prevent microbial ingress, and implementing real-time contamination detection systems are essential. Techniques include clean-in-place (CIP), steam-in-place (SIP), and strict aseptic sampling. Preventing contamination preserves product integrity and minimizes costly downtime.
3. Product Purity and Yield
High-purity output is vital for pharmaceuticals and bioproducts. Downstream processing like filtration, chromatography, and ultrafiltration must efficiently separate the desired product from host cell proteins, DNA, and other contaminants. Designing optimized purification trains that maintain high yield while meeting regulatory-grade purity is a key engineering challenge.
4. Regulatory Compliance
Biochemical engineers must navigate a complex regulatory landscape governed by agencies such as the FDA in the U.S., EMA in Europe, and other local regulators. Meeting Good Manufacturing Practice (GMP) standards requires rigorous validation of every process step, detailed documentation, traceability of raw materials, and robust quality control. Engineers also must prepare for frequent audits and adapt to evolving regulations.
5. Sustainability and Environmental Impact
Scaling bioprocesses sustainably means reducing energy consumption and minimizing waste. Engineers are challenged to design processes that recycle water and solvents, reduce carbon footprint, and utilize renewable feedstocks. Implementing green engineering principles—such as closed-loop systems, biocatalysis, and biodegradable waste streams—helps align production with environmental goals.
Additional Considerations
Process Monitoring and Control: Advanced sensors, process analytical technology (PAT), and automation systems are required to maintain tight control over fermentation parameters like dissolved oxygen, temperature, and pH.
Cost vs. Quality Trade-Offs: Balancing production costs with product quality and regulatory compliance demands careful process economics and lifecycle analysis.
Workforce Expertise: Skilled personnel trained in bioprocess engineering, regulatory affairs, and quality assurance are essential for industry success.
Looking Ahead
To address these challenges, the field is moving towards continuous bioprocessing, modular production platforms, and integration of AI-driven monitoring. These advancements promise to make biomanufacturing more flexible, efficient, and sustainable without compromising product quality.
Learn more about regulatory frameworks at FDA GMP Guidance and explore innovations in sustainable bioprocessing at Journal of Cleaner Production.
Future Directions in Biochemical Engineering
Personalized Medicine
Biochemical engineering is paving the way for precision therapeutics tailored to the genetic profile of each patient. Personalized medicine involves the use of genomic data, metabolic profiling, and patient-specific biomarkers to design customized drug formulations, dosages, and delivery methods. This approach reduces adverse effects and enhances therapeutic efficacy. Engineers are developing flexible bioprocess platforms that can quickly adjust production lines to manufacture small batches of individualized biologics.
One notable development is the use of microfluidic chips for patient-specific diagnostics and drug screening, enabling rapid analysis and on-demand drug synthesis. Integration with wearable biosensors and AI-driven diagnostics is also expanding the feasibility of decentralized, patient-specific treatments.
Explore more about these innovations at NCBI – Personalized Medicine.
Carbon Capture and Bio-conversion
As climate change intensifies, biochemical engineers are turning to engineered microbes capable of capturing and converting carbon dioxide into fuels, polymers, and fine chemicals. This approach, known as microbial carbon fixation, utilizes photosynthetic organisms like cyanobacteria or synthetic autotrophs that consume CO₂ as a feedstock.
Advanced genetic engineering techniques, including CRISPR and metabolic modeling, are enabling microbes to be reprogrammed for higher conversion efficiency and selectivity. The use of biocatalysts in carbon mineralization processes also presents new ways to create value-added products from captured emissions.
Read more about cutting-edge carbon bioconversion at Nature Reviews – Microbial Carbon Engineering.
Biofabrication and Tissue Engineering
The integration of biochemical engineering and 3D bioprinting is revolutionizing the creation of functional tissues and organs. Biofabrication involves the layer-by-layer deposition of bio-inks containing living cells and supportive biomaterials to construct structures that mimic human tissue architecture.
This innovation holds promise for producing skin grafts, vascular tissues, and even entire organs for transplantation. Engineers are working on vascularization techniques, scaffold optimization, and cell viability during printing to ensure structural and functional fidelity.
Future directions include smart bioinks that respond to stimuli, dynamic scaffolds with embedded growth factors, and on-demand organ printing tailored to individual patients using autologous cells.
Smart Bioreactors
Smart bioreactors are equipped with sensors, data analytics, and artificial intelligence (AI) to monitor and adjust bioprocess parameters in real-time. These systems enhance efficiency, reduce human error, and support predictive maintenance.
Internet of Things (IoT) integration allows remote access to performance metrics like pH, oxygen levels, temperature, and nutrient concentrations. Machine learning algorithms can detect patterns and optimize fermentation cycles for maximum yield and minimal waste.
Applications range from pharmaceutical production to bioenergy generation. The shift toward modular, cloud-connected bioreactor systems is enabling more scalable and decentralized biomanufacturing facilities.
Waste-to-Energy Technologies
Biochemical engineering is at the forefront of converting organic waste into renewable energy through microbial fermentation and anaerobic digestion. Engineered bacteria and archaea are optimized to break down agricultural residues, food waste, and sewage into methane, hydrogen, and bioethanol.
New metabolic pathways are being developed to enhance substrate utilization and increase energy output. These innovations reduce landfill burden, lower greenhouse gas emissions, and contribute to a circular bioeconomy.
Future systems will integrate waste-to-energy units with urban infrastructure, making cities more self-sufficient and resilient in energy production.
Why Study Biochemical Engineering
Engineering Biological Processes
Biochemical engineering involves applying chemical engineering principles to biological systems. Students learn to design bioreactors and optimize fermentation processes. These systems are used to produce biofuels, vaccines, and enzymes.
Understanding Microorganisms and Cells
Students study how bacteria, yeast, and mammalian cells can be used for industrial production. They explore cell metabolism, growth kinetics, and genetic engineering. This supports innovation in synthetic biology and biotechnology.
Scale-Up and Manufacturing
The course covers challenges in scaling lab processes to industrial production. Students learn how to maintain product quality, yield, and consistency. This prepares them for roles in pharmaceutical and bioprocessing industries.
Environmental and Health Applications
Biochemical engineers contribute to pollution control, water treatment, and health care. Students learn to create sustainable solutions with minimal environmental impact. This reinforces engineering’s role in solving societal challenges.
Growing Demand and Career Versatility
With the rise of biotech and green industries, demand for biochemical engineers is increasing. Students are equipped for careers in R&D, production, or regulatory roles. The field combines innovation with meaningful impact.
Biochemical Engineering: Conclusion
Biochemical Engineering is at the forefront of technological innovations that harness biological systems for industrial and healthcare solutions. By integrating biology with chemical engineering principles, this field has revolutionized drug manufacturing, renewable energy production, and environmental sustainability. As the world seeks greener, more efficient solutions, biochemical engineers will continue to lead advancements in biotechnology, shaping a more sustainable and healthier future.
Biochemical Engineering – Frequently Asked Questions (FAQ)
1. Is biochemical engineering more about biology or more about chemical engineering?
It is genuinely both. You work with cells, enzymes and DNA, but you also design reactors, separation trains and control systems. A biochemical engineer thinks like a process engineer while understanding how living systems behave and what they need to stay healthy and productive.
2. How is biochemical engineering different from studying pure biotechnology?
Biotechnology often focuses on laboratory-scale biology, such as cloning genes or characterising cell lines. Biochemical engineering adds scale-up, process design, economics and regulatory constraints, asking: “How do we run this safely and efficiently in a 10,000-litre plant, not just a 1-litre flask?”
3. What kinds of industries employ biochemical engineers?
Biochemical engineers are employed in biopharmaceutical and vaccine manufacturing, food and beverage fermentation, industrial biotechnology, biofuels, bioplastics, diagnostics and environmental bioremediation. Many also work in start-ups that apply synthetic biology to create new products and materials.
4. Do I need strong wet-lab skills to study biochemical engineering?
Wet-lab skills are useful, especially for working with microbes, cell cultures and analytical assays. However, you also need strong quantitative skills in mathematics, modelling and process analysis. Programmes usually aim to develop both practical laboratory experience and rigorous analytical thinking.
5. What is the role of bioreactors in real industrial settings?
In industry, bioreactors are the “heart” of many processes. They provide carefully controlled conditions for cells or enzymes to convert cheap feedstocks into high-value products. Biochemical engineers design, scale up and control these bioreactors so that productivity, quality and product consistency are maintained.
6. Why is downstream purification often the most expensive part of a bioprocess?
Bioproducts such as proteins or metabolites are usually present at low concentrations in complex broths. Separating them gently without loss of activity requires multiple steps and specialised equipment. Each separation stage adds cost, so biochemical engineers carefully design purification trains to balance purity, yield and economy.
7. How do biochemical engineers engage with genetic engineering and synthetic biology?
They collaborate with molecular biologists to design strains and cell lines, then test how these behave in bioreactors and at pilot scale. Biochemical engineers use metabolic models and process data to suggest further genetic modifications that improve yield, robustness or the use of cheaper raw materials.
8. What are the main challenges in scaling up bioprocesses?
Major challenges include providing sufficient oxygen and nutrients, avoiding damaging shear forces, controlling heat removal, preventing contamination and keeping product quality consistent between batches. Scale-up often requires iterative pilot studies and careful modelling before full industrial deployment.
9. Are there ethical issues I should be aware of in biochemical engineering?
Yes. Topics such as the release of genetically modified organisms, gene editing in humans or animals, access to lifesaving biologic drugs and the environmental impact of large-scale biomass use all raise ethical questions. Responsible biochemical engineers engage with regulations and public concerns when developing new technologies.
10. How does biochemical engineering contribute to global health and sustainability?
Biochemical engineering enables large-scale vaccine production, more accessible biopharmaceuticals, cleaner biofuels, biodegradable materials and processes that use renewable feedstocks. These contributions have direct impacts on public health, climate goals and more sustainable patterns of production and consumption.
Biochemical Engineering: Review Questions and Answers
-
What is biochemical engineering, and how does it extend traditional chemical engineering?
Answer: Biochemical engineering is a branch of chemical engineering that focuses on processes involving living cells, enzymes and other biological molecules. It extends traditional chemical engineering by combining reaction engineering, transport phenomena and process design with microbiology, biochemistry and molecular biology to produce bioproducts such as vaccines, therapeutic proteins, biofuels, enzymes and fermented foods at industrial scale. -
How are fermentation processes used in industrial biochemical engineering?
Answer: In industrial fermentation, microorganisms such as bacteria, yeast or fungi convert inexpensive substrates into valuable products. Biochemical engineers design and operate fermenters to control temperature, pH, oxygen supply, agitation and nutrient availability so that cells grow healthily and produce high yields of products like antibiotics, organic acids, alcohols, vitamins and enzymes in a reproducible way. -
What roles do enzymes play in biochemical processes, and how are they engineered for industry?
Answer: Enzymes act as highly specific biological catalysts, speeding up reactions under mild conditions and reducing the need for harsh chemicals. In industry they are used in drug synthesis, food processing, detergents and biofuel production. Biochemical engineers stabilise enzymes by immobilising them on solid supports, tailoring operating conditions and working with scientists who modify enzyme structures to improve activity, selectivity and robustness. -
What are bioreactors, and which operating conditions are critical to their performance?
Answer: Bioreactors are vessels designed to house biological reactions involving cells or enzymes in a controlled environment. Key operating conditions include temperature, pH, dissolved oxygen, nutrient concentrations, mixing intensity and sterility. Biochemical engineers choose appropriate bioreactor types—such as stirred-tank, airlift or packed-bed designs—and control strategies to maintain these conditions and maximise productivity while protecting biological activity. -
Why is downstream processing essential in biochemical engineering, and what major steps does it involve?
Answer: Downstream processing is essential because products must be separated and purified from complex broths before they are safe and useful. Major steps include cell removal (for example by centrifugation or filtration), product capture (such as extraction or precipitation), purification (often using chromatography or membrane processes) and final polishing and formulation (such as crystallisation, drying or sterile filtration) to reach the required purity and stability. -
What is metabolic engineering, and how is it applied to improve bioprocesses?
Answer: Metabolic engineering involves systematically modifying cellular pathways to redirect flux toward desired products or to introduce new capabilities. Biochemical engineers work with genetic tools to upregulate beneficial enzymes, knock out competing routes and adjust regulatory networks. This can lead to higher product titres and yields, the ability to use cheaper feedstocks, or the biosynthesis of entirely new chemicals that were not naturally produced by the organism. -
What challenges arise when scaling up bioprocesses from laboratory flasks to industrial plants?
Answer: Scaling up must preserve cell health and product quality while volumes increase by many orders of magnitude. Challenges include achieving uniform mixing, supplying enough oxygen, controlling heat generation, avoiding damaging shear forces and maintaining sterile conditions. Biochemical engineers use scale-up criteria, computational models and pilot-scale tests to adjust equipment design and operating conditions so that large-scale performance remains reliable. -
How does synthetic biology complement biochemical engineering, and what applications does it enable?
Answer: Synthetic biology provides tools for designing new genetic circuits and constructing organisms with customised metabolic pathways. When combined with biochemical engineering, it enables the creation of engineered microbes that produce complex pharmaceuticals, advanced materials, biofuels and specialty chemicals. It also supports applications such as biosensors, programmed cells for environmental remediation and novel therapeutic strategies. -
What ethical and biosafety issues must biochemical engineers consider, especially when using genetically modified organisms?
Answer: Biochemical engineers must consider the potential ecological impact of GMOs, the risk of unintended gene transfer, and the consequences of accidental release into the environment. They also engage with ethical questions around gene editing in humans, animals and crops, as well as fairness in access to bio-based medicines and technologies. Compliance with biosafety regulations, robust containment strategies and transparent communication with society are key responsibilities. -
Why are advances in bioseparation and purification technologies so important for biochemical engineering?
Answer: Many bioproducts—especially therapeutic proteins and vaccines—must meet extremely high purity standards, and purification steps often dominate the cost of production. Advances in chromatography media, membrane technologies, affinity ligands and novel separation schemes improve selectivity, reduce processing times and lower costs. These improvements are crucial for making biologic therapies and other bioproducts more affordable and widely available.
Thought-Provoking Questions with Detailed and Elaborate Answers on Biochemical Engineering
How do microorganisms contribute to the production of biofuels, and what challenges do biochemical engineers face in optimizing these processes?
- Answer: Microorganisms, such as bacteria, yeast, and algae, convert organic substrates into biofuels like ethanol, biodiesel, and biogas through fermentation and other metabolic pathways. Biochemical engineers must optimize conditions like pH, temperature, and nutrient availability to enhance yield. Challenges include ensuring cost-effective feedstock, improving microorganism efficiency through genetic engineering, and scaling processes while maintaining sustainability.
In what ways can biochemical engineering help address global food security issues?
- Answer: Biochemical engineering enables the production of high-quality, protein-rich food additives through fermentation and microbial synthesis. Processes like single-cell protein production and enzyme-mediated food preservation extend the shelf life and nutritional value of food. By designing bioprocesses to convert agricultural waste into edible products, engineers contribute to reducing food waste and ensuring sustainable food sources.
What are the environmental benefits of bioplastics, and how can biochemical engineers improve their production?
- Answer: Bioplastics, derived from renewable biomass sources like corn or sugarcane, reduce dependence on petroleum-based plastics and minimize environmental pollution. Biochemical engineers enhance production by optimizing microbial fermentation pathways to produce biopolymers like polylactic acid (PLA) and polyhydroxyalkanoates (PHA). Challenges include reducing production costs and improving material properties like durability and biodegradability.
How can metabolic engineering be used to develop microbes capable of producing pharmaceuticals?
- Answer: Metabolic engineering involves modifying the metabolic pathways of microorganisms to enhance the production of target molecules like antibiotics, insulin, or vaccines. Biochemical engineers use tools such as CRISPR and gene editing to insert or delete genes, optimize metabolic flux, and increase yield. For example, engineered E. coli or yeast strains are widely used in producing recombinant proteins and therapeutic enzymes.
What role does enzyme immobilization play in industrial bioprocesses, and how does it improve process efficiency?
- Answer: Enzyme immobilization involves attaching enzymes to solid supports, allowing their reuse in industrial processes such as drug synthesis or food production. This improves process efficiency by reducing enzyme loss, enhancing stability, and enabling continuous operation in reactors. Biochemical engineers design supports that maintain enzyme activity while optimizing cost and scalability.
How do bioreactor designs impact the scalability of bioprocesses?
- Answer: Bioreactors provide a controlled environment for biological reactions. Their design impacts scalability by influencing parameters like oxygen transfer, mixing, and temperature control. For example, stirred-tank bioreactors are commonly used for microbial processes, while airlift bioreactors are preferred for shear-sensitive cultures. Engineers must address challenges like maintaining homogeneity and preventing contamination during scale-up.
What are the ethical implications of using genetically modified organisms (GMOs) in biochemical engineering?
- Answer: Using GMOs raises concerns about environmental impact, biosafety, and public health. Biochemical engineers must ensure containment to prevent unintended gene transfer to natural ecosystems. Ethical considerations also include transparency in labeling GMO-derived products and engaging in public dialogue to address societal concerns while demonstrating the benefits of GMOs in sustainable production.
What advancements in biochemical engineering could revolutionize waste management and environmental remediation?
- Answer: Biochemical engineering offers solutions like using microbes to degrade hazardous chemicals, convert organic waste into bioenergy, and recover valuable materials like metals from e-waste. Innovations include developing microbial consortia for bioremediation and engineering pathways for enhanced pollutant degradation. These approaches promote circular economies and environmental sustainability.
How can biochemical engineers ensure sustainability in large-scale bioprocesses?
- Answer: Sustainability is achieved by minimizing resource use and waste generation. Engineers optimize feedstock selection, recycle process water, and use renewable energy sources. Process intensification techniques, such as integrating upstream and downstream steps, further enhance efficiency. Life cycle assessment tools are employed to evaluate and improve the environmental footprint of bioprocesses.
How can synthetic biology transform the future of biochemical engineering?
- Answer: Synthetic biology allows the creation of novel biological systems and synthetic pathways for producing complex molecules. It enhances biochemical engineering by enabling precision design of biosynthetic routes for high-value products like bio-based chemicals and materials. Challenges include reducing costs, ensuring biosafety, and addressing regulatory hurdles.
What strategies can biochemical engineers employ to reduce the cost of biopharmaceutical production?
- Answer: Cost reduction strategies include optimizing fermentation conditions to maximize yield, using low-cost feedstocks, and implementing continuous manufacturing techniques. Advances in cell culture technology and process automation also improve efficiency. Downstream processing innovations, such as affinity chromatography, reduce purification costs while ensuring product quality.
How can artificial intelligence and machine learning accelerate advancements in biochemical engineering?
- Answer: AI and machine learning analyze large datasets to identify patterns, optimize bioprocess conditions, and predict outcomes. Applications include designing metabolic pathways, improving bioreactor performance, and developing robust quality control systems. These tools enable faster innovation, reduce trial-and-error experiments, and enhance decision-making in complex biochemical processes.
These thought-provoking questions encourage deeper exploration of biochemical engineering’s concepts, applications, and societal impact, fostering curiosity and critical thinking.
