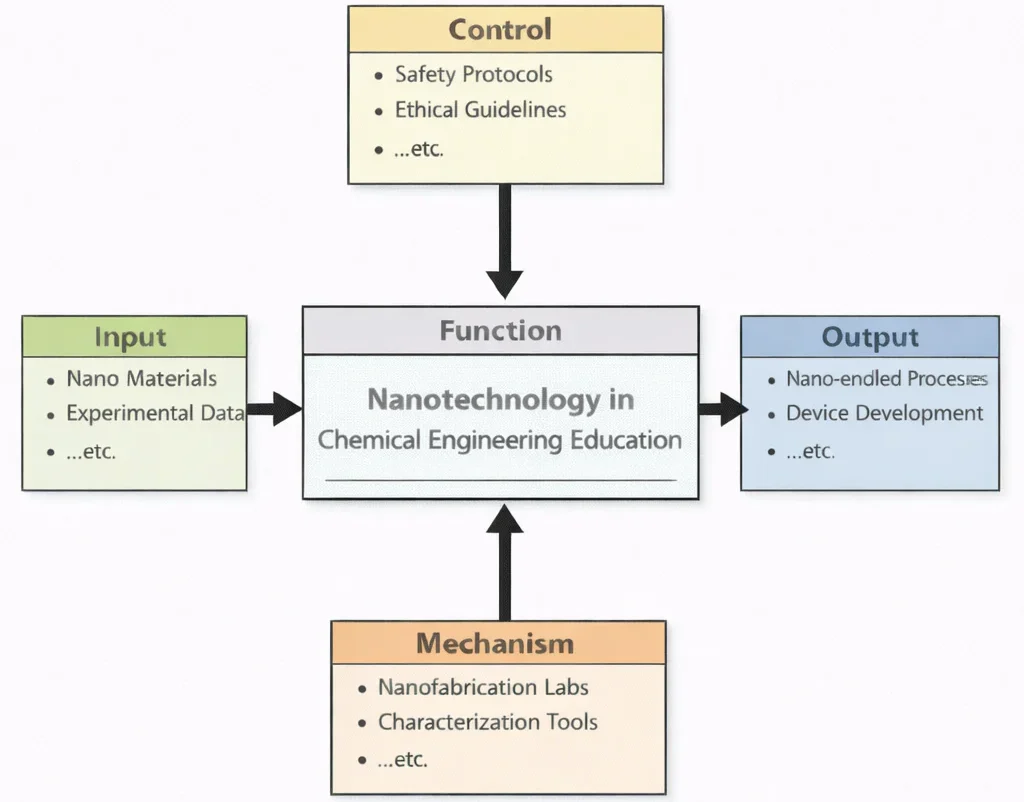
Nanotechnology in Chemical Engineering Education, viewed through this diagram, teaches students how to engineer with “smallness” without becoming careless about risk. The Inputs anchor learning in nanomaterials and experimental data, reminding students that nanoscale effects are often surprising—surface area dominates, interfaces become decisive, and tiny changes can shift performance dramatically. The Controls ensure that progress remains responsible: safety protocols protect people and the environment, while ethical guidelines shape decisions about where and how nanotechnology should be applied. The Mechanisms—nanofabrication labs and characterization tools—turn ideas into evidence, training students to build nanoscale features deliberately and verify what they actually created rather than what they hoped to create. The Outputs are graduates who can develop nano-enabled processes (such as improved catalysts, membranes, or separation methods) and contribute to device development (such as sensors or functional coatings), with the discipline to balance innovation, safety, and societal responsibility.
Nanotechnology in Chemical Engineering is revolutionizing the way materials, processes, and systems are conceived and implemented. It bridges the molecular-scale design of materials with industrial-scale performance and functionality. Building on the foundations of Chemical Engineering, this field empowers students to manipulate structures at the nanometer scale to solve problems in energy, healthcare, food production, and environmental protection.
Nanostructured catalysts have redefined reaction kinetics, making them central to advancements in Chemical Catalysis and Reaction Engineering. Similarly, breakthroughs in Chemical Energy Systems Engineering are driven by nanoscale enhancements in battery electrodes and fuel cell membranes. The interaction between nanosurfaces and macromolecules influences disciplines such as Biochemical Engineering, impacting drug delivery and biosensor development.
At the core of nanoscale innovation is a detailed understanding of structure–property relationships, which is central to Chemical Materials Engineering. Engineers use modeling tools from Computational Chemical Engineering to simulate molecular interactions, transport phenomena, and self-assembly processes. Nanoscale processing innovations are also transforming Chemical Process Engineering by enabling continuous processes with heightened precision and control.
In the realm of consumer applications, innovations developed for Food and Beverage Engineering include nanoemulsions and nanocoatings that enhance shelf life and sensory quality. New biodegradable materials, composite films, and smart packaging systems intersect with Polymer and Plastics Engineering. These developments are increasingly relevant to sectors such as Civil Engineering, where self-healing materials and advanced coatings are emerging for structural use.
The implementation of nanosensors and environmental monitors draws from techniques in Water Resources Engineering and environmental monitoring frameworks. Challenges related to risk, scale-up, and disaster prevention are guided by principles from Earthquake and Disaster Engineering. Infrastructure built with nanoreinforced materials benefits from knowledge in Structural Engineering and Geotechnical Engineering.
On the systems level, nanotechnology-driven instrumentation and automation are built using components studied in Electrical and Electronic Engineering. These include microfluidic chips and biosensors derived from Biomedical Electronics and communication modules developed in Communication Engineering. Control algorithms managing nanoscale deposition and fabrication align with principles of Control Systems Engineering.
Devices such as nano-electromechanical systems (NEMS) and high-resolution microscopy tools rely on developments in Electronics Engineering and Embedded Systems and Microelectronics. Their calibration and performance analysis benefit from the methodologies of Instrumentation and Measurement, completing the loop of integration between material, device, and process innovation.

This illustration presents nanotechnology as the “microscopic frontier” of chemical engineering. A scientist peers into a microscope, symbolizing the close observation and measurement needed to work at nano-scale dimensions. Across the scene, floating hexagonal grids, molecular structures, and particle-like textures suggest nanoparticles, surface chemistry, and engineered materials—where small changes in size and shape can drastically alter properties. On the lab benches, compact robotic devices and precision instruments imply automated experimentation, microfabrication, and high-accuracy handling of tiny samples. In the background, translucent screens and data panels evoke simulation, characterization (such as spectroscopy or microscopy analysis), and process optimization. Overall, the image captures how nanotechnology supports chemical engineering goals—creating advanced catalysts, improving separations and membranes, enhancing drug delivery or coatings, and enabling cleaner, more efficient industrial processes through materials engineered at the smallest scales.
Nanotechnology for Chemical Engineering – Invisible FAQ
- How does nanotechnology extend what traditional chemical engineering can do?
- Nanotechnology lets chemical engineers design materials and devices by controlling structure at the 1–100 nm scale. This enables catalysts with far higher activity, functional coatings with special optical or barrier properties, and energy materials with faster charge and mass transport than would be possible using only bulk-scale design.
- What makes nanomaterials behave differently from bulk materials?
- At the nanoscale, a very high fraction of atoms sit at or near the surface and quantum effects become important. These factors change melting points, colour, conductivity, chemical reactivity and mechanical strength, giving nanomaterials properties that do not simply scale down from the bulk solid.
- Which classes of nanomaterials are most relevant to chemical engineering?
- Chemically engineered nanomaterials include metal and metal-oxide nanoparticles, carbon-based nanostructures (such as nanotubes and graphene), nanoporous materials like zeolites and MOFs, and nano-structured polymers and composites. Each class can be tuned for catalysis, separations, sensors or energy systems.
- What is the difference between top-down and bottom-up nanomaterial synthesis?
- Top-down methods carve or break bulk solids down into nanoscale structures using milling, etching or lithography. Bottom-up methods assemble nanostructures from atoms or molecules via precipitation, sol–gel chemistry, self-assembly or vapour-phase deposition. Bottom-up routes often offer finer control over composition and morphology.
- How are nanocatalysts designed for industrial reaction engineering?
- Nanocatalysts are engineered by controlling particle size, shape, composition and support interactions to maximise the number and quality of active sites. Chemical engineers also design reactors that ensure good contact between nanocatalyst surfaces and reactants while minimising sintering, poisoning and mass-transfer limitations.
- In what ways does nanotechnology support cleaner and more efficient energy systems?
- Nanostructured electrodes increase the capacity and cycling stability of batteries and supercapacitors, while nano-engineered catalysts improve fuel cell performance and hydrogen production. Quantum dots and other nano-absorbers enhance light harvesting in solar cells, supporting higher efficiencies and thinner, lighter devices.
- What are key environmental and health questions around nanomaterials?
- Concerns include inhalation or ingestion risks, bioaccumulation, persistence in soil and water, and potential toxicity to cells and ecosystems. Engineers must consider safe-by-design principles, exposure control, end-of-life management and regulatory guidance when developing nano-enabled products and processes.
- How can nanotechnology help with pollution control and remediation?
- Nanomaterials can be used as highly active adsorbents, photocatalysts or reactive particles for removing heavy metals, dyes, organic pollutants and greenhouse gases from air and water. Their large surface area and tunable surface chemistry make them effective for capturing and transforming contaminants.
- What makes scale-up of nanomaterial production technically challenging?
- When moving from lab to plant, it is difficult to maintain tight control over particle size distributions, surface chemistry and dispersion state. Agglomeration, fouling of equipment, safety of nanopowder handling and cost of precursors also complicate industrial-scale implementation.
- Which skills are important for students interested in nanotechnology within chemical engineering?
- Students benefit from strong foundations in thermodynamics, transport phenomena and reaction engineering, combined with solid-state chemistry, surface science, characterisation techniques (such as TEM, SEM and spectroscopy) and basic data science for analysing complex structure–property relationships.
- Chemical Engineering topics:
- Chemical Engineering – Overview
- Chemical Process Engineering
- Chemical Catalysis & Reaction Engineering
- Chemical Materials Engineering
- Chemical Energy Systems Engineering
- Polymer & Plastics Engineering
- Nanotechnology in Chemical Engineering
- Biochemical Engineering
- Food & Beverage Engineering
- Computational Chemical Engineering
Table of Contents
Core Concepts in Nanotechnology for Chemical Engineering
Nanomaterials Synthesis and Fabrication
Nanomaterials synthesis forms the backbone of advanced chemical engineering, combining precise control over size, shape, and composition. By manipulating materials at the atomic or molecular level, engineers create structures with unparalleled properties.
Approaches to Fabrication
- Top‑Down Techniques: This method involves reducing bulk materials into nanoscale particles via mechanical milling, lithographic patterning, and etching processes.
- Bottom‑Up Methods: Building nanostructures atom by atom or molecule by molecule through self-assembly, chemical vapor deposition, and atomic layer deposition.
Classification of Nanomaterials
- Nanoparticles: Metallic, metal-oxide, and polymeric forms used in catalysis and drug delivery.
- Nanotubes: Structures like carbon nanotubes and boron nitride tubes ideal for strength and conductivity enhancements.
- Nanowires: Thin, conductive strands used in sensors and electronics.
- Quantum Dots: Semiconductor particles emitting specific wavelengths for imaging and display uses.
- Nanocomposites: Hybrid assemblies mixing nanoparticles with bulk matrices for tough, functional materials.
Nanostructured Catalysts
Nanoscale catalysts revolutionize chemical reactions by dramatically increasing surface-to-volume ratio, enabling faster, more selective, and energy-efficient processes.
- Benefits: They enhance reaction rates, minimize by-products, and lower temperature/pressure needs.
- Applications: Vital in green chemistry, renewable fuel synthesis, and environmental cleanup.
Surface Functionalization and Self‑Assembly
Tuning surface chemistry of nanomaterials is crucial to achieving specific interactions, from binding biomolecules to resisting corrosion.
- Self‑Assembled Monolayers (SAMs): Organothiols form organized layers on metals for sensors or coatings.
- Ligand Attachment: Attaching specific molecules to nanoparticles enables targeted drug delivery or biosensing.
- Use Cases: Includes biosensor surfaces, precision drug carriers, and protective coatings.
Nanofluidics and Nano‑Scale Transport Phenomena
Manipulating fluids at the nanoscale supports innovations in diagnostics, filtration, and controlled release systems.
- Lab‑on‑a‑Chip Devices: Miniaturized systems for point-of-care testing through micro- and nano-fluid channels.
- Water Purification: Nanoporous membranes offer high selectivity and efficiency in filtration applications.
- Targeted Drug Delivery: Nanofluidic reservoirs enable precise dosing for advanced therapies.
Characterization and Modeling of Nanostructures
Advanced characterization and computational tools are essential for analyzing nanomaterials’ behavior and properties.
- Microscopy Techniques: TEM, AFM, and scanning electron microscopy reveal nanostructure details.
- Spectroscopy Tools: Includes Raman, infrared, and UV–Vis spectroscopy for chemical identification.
- Computational Modeling: Quantum chemistry, atomistic simulation, and multiscale modeling tools provide property and reaction predictions.
Safety, Ethics, and Regulation
As applications expand, addressing safety and regulatory aspects is increasingly important.
- Health & Safety: Studies on nano-toxicology evaluate risks of inhaled or ingested nanomaterials.
- Environmental Effects: Research investigates how nanoscale particles interact within ecosystems.
- Regulatory Frameworks: Agencies like EPA and EU bodies create guidelines for safe manufacturing and disposal.
Key Applications of Nanotechnology in Chemical Engineering
Drug Delivery Systems
Nanocarriers revolutionize therapeutic delivery with precision control and improved patient outcomes:
- Liposomes: Spherical vesicles that encapsulate drugs, providing controlled release and reduced toxicity when combined with phospholipid bilayers.
- Polymeric Nanoparticles: Made of biodegradable materials like PLA or PLGA, ideal for targeted delivery.
- Dendrimers: Highly branched macromolecules enabling precise drug loading and multifunctional surface modification with peptide groups.
- Carbon Nanotubes (CNTs): Functionalized to penetrate cells, they support gene therapy and targeted oncology treatments.
Advantages: Enhanced bioavailability, targeted action, minimized side effects, and controlled release profiles make them powerful tools in modern pharmaceutics.
Water Purification and Treatment
- Nanofiltration Membranes: Nanoscale pores remove contaminants like heavy metals, leveraging membrane technology.
- Silver Nanoparticles: Their antimicrobial behavior supports water disinfection.
- Iron Oxide Nanoparticles: Effective at arsenic removal and heavy metal adsorption.
- Photocatalysis: Titanium dioxide (TiO₂) nanoparticles use UV light to break down organic pollutants in advanced oxidation processes.
Energy Production and Storage
- Nano‑enhanced Lithium‑Ion Batteries: Silicon and graphene anodes boost capacity and enable fast charging with graphene.
- Solid-State Batteries: Incorporate nanostructured electrolytes for enhanced safety and conductivity.
- Fuel Cells: Platinum and alternative metal nanoparticles improve electrocatalytic efficiency.
- Supercapacitors: Carbon nanomaterials enhance energy density for rapid charge/discharge cycles.
- Solar Cells: Quantum dots and perovskite nanomaterials push the boundaries of photovoltaic technology.
Advanced Sensors and Detection Systems
- Metal Oxide Nanowires: Highly sensitive to gases using gas detection techniques.
- Graphene & Gold‑Based Biosensors: Detect biomolecules for diagnostics and environmental monitoring.
- Nanosensors in Process Control: Enable precise real‑time monitoring in industrial settings.
Catalysis and Reaction Engineering
- Nanocatalysts: Platinum, gold, iron oxide particles accelerate chemical reactions with high selectivity.
- Nanoscale Photocatalysts: Support green chemistry by degrading pollutants under light.
- Electrocatalysis: Nanomaterials enhance water splitting and fuel cell efficiency.
Environmental Remediation
- Nanoremediation: Iron nanoparticles remove groundwater pollutants via contaminant degradation.
- CNTs & Graphene Adsorbents: Useful in treating organic and oil spills due to high surface area.
- Photocatalytic Air Purifiers: TiO₂-based systems break down airborne toxins efficiently.
Food and Agriculture
- Nano-Encapsulation: Protects and releases nutrients in food, enhancing stability and bioavailability.
- Nanosensors in Food Chains: Detect pathogens and spoilage in real-time supply chain conditions.
- Nano‑Fertilizers: Controlled-release fertilizers promote efficient nutrient delivery and reduce runoff.
Emerging Technologies in Nanotechnology for Chemical Engineering
Graphene and 2D Materials
- Properties:
- Exceptional strength, thermal conductivity, and electrical properties.
- Applications:
- Flexible electronics, advanced composites, and supercapacitors.
Graphene and other two-dimensional materials have revolutionized material science by offering atomic-level thinness with unprecedented performance characteristics. These materials possess a unique combination of tensile strength, electrical conductivity, and transparency, which has spurred innovations in flexible electronics and energy storage systems. In chemical engineering, graphene membranes are being developed for ultra-efficient filtration processes, enabling desalination and solvent recovery with significantly reduced energy input. Moreover, the incorporation of 2D materials into polymers enhances barrier properties, extending shelf life in food packaging and increasing chemical resistance in industrial coatings.
Quantum Dots
- Definition:
Semiconductor nanoparticles that emit light at specific wavelengths. - Applications:
- Solar cells, LEDs, and bio-imaging.
Quantum dots are nanoscale semiconductors with size-dependent optical and electronic properties, making them ideal for a range of optoelectronic applications. Their tunable emission spectra allow for enhanced imaging precision in biomedical diagnostics and targeted drug delivery systems. In the field of renewable energy, quantum dots are enhancing photovoltaic performance by absorbing a broader range of the solar spectrum. These advances in solar technology are supported by quantum dot-based solar cells now under active development. The unique photoluminescence of quantum dots also enables their integration into advanced sensors for chemical process monitoring and environmental pollution detection.
Carbon Nanotubes (CNTs) and Nanofibers
- Properties:
- High mechanical strength and electrical conductivity.
- Applications:
- Structural reinforcement, energy storage, and electronic devices.
Carbon nanotubes (CNTs) and nanofibers are among the most extensively studied nanomaterials due to their remarkable mechanical, thermal, and electronic characteristics. These structures have found applications in strengthening polymers, reducing weight in composite materials, and enhancing performance in capacitors and lithium-ion batteries. Their high surface area and electrical conductivity make CNTs ideal for creating conductive inks and thermal interface materials in electronics manufacturing. In chemical engineering, CNTs are also being used as supports for heterogeneous catalysts, significantly improving reaction efficiency and selectivity in petrochemical and pharmaceutical processes.
Nanostructured Coatings
- Applications:
- Anti-corrosion, anti-fouling, and self-cleaning surfaces.
- Materials:
- Superhydrophobic coatings and antimicrobial surfaces.
Nanostructured coatings provide a powerful layer of functional protection for a wide variety of surfaces. These coatings are designed to respond dynamically to environmental conditions, offering benefits such as self-healing, anti-microbial action, and resistance to corrosion and fouling. Superhydrophobic coatings, for instance, mimic the lotus leaf effect, causing water to bead and roll off, carrying contaminants with it. This property is especially beneficial in the food and beverage, marine, and biomedical industries. Antimicrobial nanocoatings using silver or copper nanoparticles are being deployed in hospitals and public spaces to reduce pathogen transmission and support infection control efforts.
Integrating Emerging Nanotechnologies into Industrial Processes
The translation of these nanotechnologies into real-world industrial processes requires a multidisciplinary approach involving chemical engineers, materials scientists, and manufacturing experts. One of the greatest challenges lies in scaling up production while preserving nanoscale precision. Advances in nanomanufacturing, combined with national nanotechnology initiatives, are helping to bridge this gap by funding research and supporting pilot programs. These efforts are critical for transitioning laboratory breakthroughs into commercially viable solutions that enhance the performance and sustainability of chemical manufacturing systems worldwide.
Challenges in Nanotechnology for Chemical Engineering
Scale-Up and Manufacturing:
- Transitioning from lab-scale to industrial-scale production of nanomaterials.
While nanotechnology has yielded transformative innovations at the laboratory level, one of the most pressing challenges is the reliable and cost-effective scale-up of nanomaterials for commercial manufacturing. Processes that work well in a controlled lab environment often face technical bottlenecks when scaled, such as achieving uniform particle size, avoiding agglomeration, and maintaining product consistency at high volumes. Manufacturing processes must be adapted to ensure reproducibility and scalability while minimizing energy consumption and material waste. Chemical engineers are working to integrate continuous-flow reactors, advanced dispersion technologies, and precision control systems to bridge the lab-to-industry gap. Additionally, facilities must often be retrofitted or newly constructed to handle nanoscale production under safe and clean conditions.
Toxicity and Environmental Impact:
- Assessing and mitigating potential health and environmental risks.
The environmental and health implications of engineered nanomaterials (ENMs) remain an area of active investigation. Many nanomaterials, including carbon nanotubes and certain metal oxides, exhibit unique surface properties and high reactivity that may pose biological risks when inhaled, ingested, or released into ecosystems. Assessing toxicity is particularly complex because conventional toxicology methods may not fully capture the behavior of particles at the nanoscale. Scientists are developing new risk assessment protocols for nanomaterials, including in vitro testing, life cycle analysis, and environmental dispersion modeling. As awareness grows, regulatory bodies and industries are focusing on sustainable nanotechnology design, using green synthesis methods and biodegradable components to reduce long-term ecological footprint.
Regulatory and Ethical Issues:
- Establishing safety standards and regulations for nanomaterials.
The regulatory landscape for nanotechnology is still evolving, and current frameworks often struggle to keep pace with the rapid development of new materials and applications. Establishing standardized definitions, classification systems, and labeling requirements for nanomaterials is crucial to ensure consumer safety and industry accountability. The absence of harmonized global standards can also hinder international collaboration and trade. Ethical concerns surrounding the deployment of nanotechnologies—such as informed consent for nano-enabled medical treatments, data privacy in nanosensors, and the potential misuse of nanomaterials—must also be addressed. Institutions like the World Health Organization are increasingly engaged in developing ethical guidelines and supporting regulatory capacity building for countries integrating nanotech into healthcare and manufacturing systems.
Cost-Effectiveness:
- Reducing production costs for widespread commercial applications.
Despite the promise of enhanced performance and functionality, many nano-enabled products remain economically unfeasible for large-scale adoption due to high production costs. Synthesis methods involving rare metals, cleanroom environments, or energy-intensive processes contribute to high capital and operational expenditures. For nanotechnology to become viable in mainstream chemical engineering, innovations must be paired with low-cost manufacturing strategies—such as solution-based synthesis, self-assembly, and additive manufacturing. Moreover, achieving economies of scale will require demand aggregation, standardized components, and modular processing units that can be easily integrated into existing industrial workflows. Public–private partnerships, startup incubators, and open innovation networks are also crucial in lowering market entry barriers and accelerating commercialization.
Interdisciplinary Knowledge Gaps:
Nanotechnology operates at the intersection of chemistry, physics, biology, and materials science. Engineers in the chemical field may not always possess the specialized knowledge needed to predict or control nano-specific interactions such as quantum confinement or surface plasmon resonance. Similarly, toxicologists, regulators, and policy-makers often require enhanced familiarity with nanoscale phenomena to make informed decisions. Bridging these gaps requires the development of interdisciplinary training programs, integrated curricula, and collaborative research centers. Only through the alignment of science, engineering, and policy can nanotechnology fulfill its potential in advancing safer, more sustainable industrial processes.
Future Directions in Nanotechnology for Chemical Engineering
Circular Economy and Sustainability:
- Developing biodegradable and recyclable nanomaterials.
The integration of nanotechnology with the principles of the circular economy is poised to revolutionize material lifecycles in chemical engineering. Instead of relying on traditional materials that generate long-term waste, researchers are developing nanomaterials that are not only functional but also environmentally benign. For example, biodegradable nanosheets and cellulose-based nanoparticles can break down naturally, reducing their ecological footprint. Innovations also include nanocomposites that can be disassembled and repurposed at the end of their useful life, facilitating material recovery and reuse. This trend aligns closely with international efforts to minimize e-waste and chemical pollution. Research funded by national nanotechnology initiatives emphasizes sustainable material sourcing and green nanomanufacturing methods. These developments will be essential for building a closed-loop economy where high-performance materials do not compromise environmental integrity.
Smart and Responsive Nanomaterials:
- Materials that adapt to environmental stimuli for dynamic applications.
A key frontier in nanotechnology is the creation of smart nanomaterials—those that dynamically respond to changes in their environment, such as pH, temperature, light, or mechanical stress. In chemical engineering, such materials can be incorporated into self-regulating coatings, targeted drug delivery systems, or adaptive filtration membranes. For instance, thermo-responsive nanogels are already being used to control drug release rates in pharmaceutical formulations. Similarly, self-healing polymers with embedded nanoparticles can restore structural integrity after damage, offering enhanced durability and performance. These adaptive capabilities are especially valuable in sectors like aerospace, automotive, and food packaging, where reliability and responsiveness are critical. The increasing functionality of these materials will redefine engineering design standards and open up new applications in responsive systems and autonomous technologies.
Nano-enabled Energy Systems:
- High-efficiency solar cells and hydrogen production technologies.
Energy generation and storage are critical global challenges, and nanotechnology offers transformative solutions across both domains. Nano-engineered materials such as quantum dots, perovskite nanocrystals, and carbon nanostructures are revolutionizing photovoltaic devices by significantly improving light absorption, charge transport, and efficiency. These advancements have the potential to make solar energy more accessible and cost-effective worldwide. Additionally, nanocatalysts are being employed to facilitate hydrogen production through water splitting and electrolysis, offering promising alternatives to fossil fuels. The surface-to-volume ratio and tunable electronic properties of nanomaterials make them ideal for boosting reaction kinetics in renewable energy processes. Research from institutions like NREL (National Renewable Energy Laboratory) continues to highlight the critical role of nanotechnology in achieving cleaner, smarter energy systems.
AI-Driven Nanomaterial Design:
- Accelerating discovery and optimization through machine learning.
Artificial intelligence (AI) is rapidly transforming the way nanomaterials are discovered, characterized, and applied in chemical engineering. By leveraging vast datasets and predictive algorithms, AI enables the accelerated identification of optimal material compositions, structures, and processing techniques. This is particularly valuable in nanotechnology, where trial-and-error synthesis is both costly and time-consuming. Machine learning models can predict properties such as thermal conductivity, catalytic activity, or biocompatibility even before a material is physically fabricated. Furthermore, AI systems integrated with high-throughput experimentation platforms are enabling real-time feedback loops, driving iterative improvement and autonomous discovery. This synergy between computational power and materials science is laying the foundation for a new era of precision engineering in which novel nanostructures are developed with unprecedented speed and accuracy.
Biomedical Integration and Targeted Therapies:
Another promising direction lies in the convergence of nanotechnology with biomedical engineering, where nanostructures are being designed for precision diagnostics and personalized medicine. Nanoscale carriers, such as liposomes, dendrimers, and metallic nanoparticles, can transport drugs directly to diseased cells, minimizing side effects and improving therapeutic outcomes. Imaging agents enhanced with nanoparticles are also enabling earlier detection of diseases like cancer, cardiovascular disorders, and neurodegenerative conditions. Researchers are now focusing on creating multifunctional nanoplatforms that combine diagnostics, therapy, and real-time monitoring in a single system. The evolution of these medical applications is supported by growing investments and ethical oversight to ensure patient safety and long-term biocompatibility.
Environmental Remediation and Sensing:
Future applications of nanotechnology are also geared toward environmental monitoring and cleanup. Nanomaterials with high surface reactivity, such as nano-iron or titanium dioxide, are being deployed for the degradation of pollutants and the removal of heavy metals from water. Simultaneously, nanosensors embedded in industrial systems or wearable devices can provide real-time data on air and water quality, emissions, and other critical parameters. These applications are vital in mitigating climate change and reducing environmental harm caused by chemical industries. As technology matures, scalable deployment of such systems will be key to ensuring cleaner ecosystems and healthier communities.
Why Study Nanotechnology in Chemical Engineering
Manipulating Matter at the Nanoscale
Nanotechnology in chemical engineering involves designing and manipulating materials at the scale of atoms and molecules. Students learn how changes at the nanoscale can lead to dramatic differences in material properties. This enables the creation of advanced materials with superior performance characteristics.
Revolutionizing Energy and Environmental Systems
Nanomaterials are critical for innovations in energy storage, solar power, and water purification. Students explore how nanoscale catalysts, membranes, and sensors contribute to cleaner, more efficient technologies. These applications support global sustainability and green engineering goals.
Advanced Tools and Characterization Techniques
The field introduces students to high-resolution imaging and surface analysis methods such as AFM, SEM, and spectroscopy. Mastery of these tools is essential for understanding nanoscale structures and ensuring quality control. These techniques are foundational for research and development in nanotechnology.
Applications in Health and Medicine
Nanotechnology enables precise drug delivery, diagnostic imaging, and biosensing. Students learn how nanoparticles interact with biological systems to improve healthcare outcomes. This knowledge contributes to the advancement of personalized medicine.
Cross-Disciplinary Innovation
This field sits at the intersection of physics, chemistry, biology, and engineering. Students develop versatile, interdisciplinary problem-solving skills. These capabilities are essential for leading innovations in next-generation technologies.
Nanotechnology for Chemical Engineering: Conclusion
Nanotechnology has emerged as a powerful enabler in chemical engineering, offering transformative potential across multiple sectors. No longer confined to the realm of theoretical science, nanoscale innovations are actively reshaping how materials are synthesized, how reactions are controlled, and how products are designed for superior performance. The ability to manipulate matter at the atomic and molecular level allows engineers to tailor properties such as strength, conductivity, and reactivity in ways that were previously unimaginable. This revolution is catalyzing a new era of engineering innovation—one marked by precision, efficiency, and sustainability.
Breakthroughs in Energy, Medicine, and the Environment
One of the most promising areas of application is in the energy sector, where nanostructured catalysts and electrodes are boosting the efficiency of hydrogen production, fuel cells, and energy storage systems. These technologies not only improve energy conversion but also contribute significantly to carbon neutrality goals. In medicine, nanocarriers and smart drug delivery platforms are enabling targeted therapy with reduced side effects, a breakthrough in personalized healthcare. Meanwhile, environmental engineers are using functionalized nanoparticles to clean up oil spills, degrade pollutants, and purify water with unprecedented precision.
Enhancing Process Efficiency at the Nanoscale
Chemical engineers are leveraging nanotechnology to enhance reaction kinetics and heat transfer in process equipment. Nanofluids, for instance, improve thermal conductivity in heat exchangers, while nano-enhanced membranes allow for more efficient separation and purification processes. These advancements not only reduce energy consumption but also lower operational costs across industries, from petrochemicals to pharmaceuticals.
Smart Materials and Intelligent Systems
The integration of nanotechnology has led to the creation of intelligent materials that respond dynamically to environmental stimuli. For example, shape-memory polymers and nanocomposites embedded with sensors can change their structure in real-time, adapting to stress or temperature variations. These smart materials are now being explored for use in flexible electronics, self-healing infrastructure, and responsive packaging systems. According to Nature Nanotechnology, research into such multifunctional materials continues to push the envelope of innovation.
Sustainability and Circular Economy Applications
Nanotechnology also plays a key role in advancing sustainable practices within chemical engineering. Nano-adsorbents and photocatalysts are improving air and water purification systems, while biodegradable nanomaterials are being developed to reduce long-term waste accumulation. Engineers are also exploring nanotech-based solutions for recycling rare earth elements and other critical resources, supporting circular economy principles in resource-constrained industries.
Expanding Research and Ethical Considerations
As research in nanotechnology grows rapidly, ethical and regulatory concerns are gaining prominence. Questions about nanotoxicology, long-term environmental impact, and human exposure require rigorous scientific and policy oversight. Chemical engineers must therefore collaborate closely with material scientists, ethicists, and regulatory agencies to ensure that nanotech innovations remain safe, responsible, and accessible to all.
In conclusion, nanotechnology is not merely an add-on to traditional chemical engineering—it is a paradigm shift. Its capacity to create novel materials and optimize chemical processes is already delivering measurable improvements in performance, sustainability, and innovation. As interdisciplinary collaboration deepens and computational tools accelerate material discovery, the influence of nanotechnology will only become more pervasive. Whether it’s in revolutionizing energy systems, transforming healthcare, or purifying the planet, this field is charting a bold and exciting course toward a smarter and more sustainable future in chemical engineering and beyond.
Nanotechnology for Chemical Engineering – Frequently Asked Questions (FAQ)
1. Why is nanotechnology interesting for chemical engineers?
Nanotechnology gives chemical engineers a new “toolbox” for tuning materials and reactions. By controlling structure at the nanoscale, you can make catalysts that are far more active, membranes that separate molecules more efficiently, and energy devices that store more charge in smaller, lighter packages.
2. Are nanomaterials just very small particles of ordinary materials?
They are small, but they are not just scaled-down versions of bulk materials. At the nanoscale, surface atoms dominate and quantum effects appear, so properties like colour, conductivity, reactivity and strength can change dramatically compared with the same material in bulk form.
3. What is the difference between top-down and bottom-up nanofabrication?
Top-down methods start with a larger piece of material and cut or grind it down to the nanoscale. Bottom-up methods assemble nanostructures from atoms or molecules. Chemical engineers use both, depending on whether they need precision patterns (often top-down) or finely controlled particles and films (often bottom-up).
4. How does nanotechnology make catalysts more effective?
Shrinking catalysts into nanoparticles exposes more surface atoms, which means more active sites per gram of material. Carefully controlling size, shape and composition can also make desired reaction pathways more favourable, improving both activity and selectivity while reducing energy use.
5. Where do we see nano-enabled energy technologies in practice?
Examples include nanostructured electrodes in lithium-ion batteries and supercapacitors, platinum nanoparticles in fuel cells, and quantum-dot or thin-film layers in advanced solar cells. These nano-features help move charge and ions more efficiently and capture light more effectively.
6. Are nanomaterials automatically unsafe because they are “so small”?
Not automatically. Some nanomaterials are relatively benign, while others may pose health or environmental risks. What matters are their composition, shape, surface chemistry, dose and route of exposure. Part of nanotechnology in chemical engineering is designing safer materials and processes from the outset.
7. How can nanotechnology support greener chemical processes?
Nano-engineered catalysts can allow reactions to run at lower temperatures and pressures, cutting energy use and emissions. Nanomaterials can also help capture pollutants, enable cleaner fuels and improve the performance of renewable energy technologies, contributing to more sustainable process designs.
8. Why is scaling up nanomaterial production difficult?
Conditions that give a narrow size distribution and clean surfaces in a small flask are harder to replicate in large reactors. Nanoparticles may agglomerate, stick to equipment or behave differently in large flows. Engineers must rethink reactor design, mixing and safety when scaling up.
9. Do chemical engineers working with nanotechnology spend all their time in cleanrooms?
Some work in cleanrooms, especially when collaborating with micro- and nano-fabrication labs. Many others focus on wet-chemistry synthesis, reactor design, characterisation or process modelling in more conventional lab or pilot-plant environments. The field is very diverse in daily working styles.
10. What kinds of careers can grow from nanotechnology in chemical engineering?
Graduates can move into areas such as catalysis and process development, batteries and fuel cells, advanced coatings, semiconductor and display manufacturing, environmental remediation, and research roles in both industry and academia that focus on tailored materials and nano-enabled technologies.
Nanotechnology for Chemical Engineering: Review Questions and Answers
Nanotechnology adds a powerful “small-scale” dimension to chemical engineering, allowing you to tune materials and reactions atom by atom. The questions below help you connect nanoscale ideas—surface area, quantum effects and structured materials—to real processes in catalysis, energy, and environmental engineering.
-
What is nanotechnology, and how is it applied in chemical engineering?
Answer: Nanotechnology is the science and engineering of materials and devices with at least one dimension in the range of roughly 1–100 nanometres. At this scale, properties can differ sharply from the bulk. Chemical engineers use nanotechnology to design high-performance catalysts, engineer membranes and coatings, and create advanced materials for energy storage, sensors and environmental remediation, all by controlling structure at the nanoscale. -
How do nanomaterials differ from their bulk counterparts in terms of chemical and physical properties?
Answer: Nanomaterials have a much higher surface area-to-volume ratio, so a large fraction of their atoms are exposed at the surface, where reactions occur. They may also display quantum size effects that alter optical, electrical and magnetic behaviour. As a result, properties such as reactivity, mechanical strength, colour, catalytic activity and conductivity can be quite different from those of the same material in bulk form. -
What are common approaches used to synthesise nanomaterials in chemical engineering?
Answer: Nanomaterials are typically prepared using either top-down or bottom-up strategies. Top-down approaches start with bulk solids and create nanoscale features through milling, etching or lithography. Bottom-up approaches assemble nanostructures from atoms or molecules via routes such as chemical vapour deposition, sol–gel chemistry, precipitation, self-assembly or templating. The chosen route depends on the desired composition, structure, morphology and scalability. -
How has nanotechnology improved catalytic processes in chemical reaction engineering?
Answer: Nano-catalysts offer very high surface areas and tunable active sites, which can greatly increase reaction rates and selectivity. By controlling particle size, shape and composition, engineers can favour specific reaction pathways and operate at lower temperatures and pressures. For example, noble-metal nanoparticles on tailored supports enable efficient hydrogenation, oxidation and fuel-cell reactions with reduced precious metal usage and lower energy consumption. -
What role does nanotechnology play in modern energy storage and conversion systems?
Answer: In batteries and supercapacitors, nanostructured electrodes provide short diffusion paths and large interfacial areas, boosting capacity, power density and cycling stability. In fuel cells, nano-engineered catalysts and membranes enhance electrochemical activity and durability. Nanomaterials such as quantum dots, perovskite nanocrystals and nanostructured thin films are also used to improve light absorption and charge transport in advanced solar cells. -
What environmental and health considerations are associated with the use of nanomaterials in chemical engineering?
Answer: Because of their small size and high reactivity, some nanomaterials may pose risks if inhaled, ingested or released into the environment. Concerns include potential toxicity to cells and organs, bioaccumulation in ecosystems and challenges in tracking and managing nanoparticle waste. Responsible nanotechnology requires exposure assessment, safe handling protocols, thoughtful product design and regulatory oversight to protect workers, consumers and the environment. -
How can nanotechnology contribute to more sustainable and environmentally friendly chemical processes?
Answer: Nanotechnology supports sustainability by enabling catalysts and membranes that reduce energy use, minimise solvent consumption and increase atom efficiency. Nanomaterials can improve the performance of renewable energy technologies, such as solar cells and hydrogen production systems, and can be tailored as highly active sorbents or photocatalysts for removing pollutants from air and water. Together, these applications help lower emissions and resource use across the process life cycle. -
What are some key challenges in integrating nanotechnology into industrial-scale chemical processes?
Answer: Major challenges include scaling up synthesis methods while retaining tight control over size and surface chemistry, preventing nanoparticle agglomeration, ensuring consistent quality between batches and managing safety during handling and transport. Cost of production and integration into existing process equipment are additional barriers. Overcoming these issues often requires new reactor designs, continuous processes and careful process intensification. -
Why does the high surface area-to-volume ratio of nanomaterials strongly influence their chemical reactivity?
Answer: As particles become smaller, the proportion of atoms located at or near the surface increases dramatically. Since most chemical reactions occur at surfaces or interfaces, this enlarged reactive area leads to higher apparent reactivity per unit mass. This is beneficial for catalysis and adsorption processes but also means that nanomaterials may oxidise, degrade or interact with their surroundings more rapidly than bulk materials. -
What future developments are likely for nanotechnology in chemical engineering?
Answer: Future directions include increasingly selective and durable nanocatalysts, smart nanomaterials that respond to temperature, pH or light, and integrated nano-enabled systems for carbon capture, water treatment and renewable energy. Advances in in-situ characterisation, computational modelling and machine learning are expected to accelerate the design of nanomaterials, while parallel progress in safety and regulation will support their responsible, large-scale use.
Nanotechnology for Chemical Engineering: Thought-Provoking Questions
1. How does the nanoscale size of materials influence their physical and chemical properties compared to their bulk counterparts?
Answer: At the nanoscale, materials exhibit unique properties due to their high surface area-to-volume ratio and quantum effects. For example, nanoparticles often display increased reactivity, strength, or electrical conductivity compared to their bulk counterparts. This is because a larger proportion of their atoms are exposed on the surface, enhancing their interaction with the surrounding environment. Quantum effects further contribute by altering electronic and optical behaviors, making nanomaterials suitable for applications like catalysis, sensors, and energy storage.
2. What challenges arise when scaling up the production of nanomaterials from laboratory settings to industrial applications?
Answer: Scaling up involves challenges like ensuring uniformity, maintaining material properties, and reducing costs. Inconsistent synthesis can lead to variations in particle size, impacting the material’s performance. Additionally, large-scale production requires robust processes that balance efficiency with safety, especially considering potential health and environmental risks. Overcoming these challenges involves investing in advanced manufacturing technologies and stringent quality control mechanisms.
3. How can nanotechnology improve the efficiency and sustainability of chemical processes?
Answer: Nanotechnology enables the design of highly efficient nanocatalysts that reduce the energy required for chemical reactions, lowering costs and carbon emissions. For example, catalysts with nanoscale structures provide more active sites for reactions, enhancing their efficiency. Furthermore, nanomaterials can aid in renewable energy production, such as developing efficient solar cells and hydrogen storage systems, contributing to sustainability.
4. In what ways can nanotechnology address environmental challenges, such as pollution and resource scarcity?
Answer: Nanotechnology offers solutions like nanomaterials that adsorb pollutants from water and air or break down contaminants through catalytic reactions. Additionally, it supports resource efficiency by enhancing the performance of renewable energy systems, such as solar panels and batteries. For instance, nanoparticles in filters can remove heavy metals from water, while nanostructured membranes enable efficient desalination.
5. What are the potential risks associated with the use of nanomaterials, and how can these risks be mitigated?
Answer: Risks include toxicity to humans and ecosystems, persistence in the environment, and unintended reactions due to high reactivity. Mitigation strategies involve conducting comprehensive risk assessments, designing safer nanomaterials, and adhering to strict safety protocols during production and disposal. Transparent regulations and ongoing research into long-term effects are also critical to ensuring safe use.
6. How do the properties of nanomaterials enable advancements in energy storage and conversion technologies?
Answer: Nanomaterials enhance the performance of batteries, supercapacitors, and fuel cells by increasing surface area for electrochemical reactions. For instance, nanostructured electrodes in lithium-ion batteries improve energy density and charging speed. Similarly, quantum dots in solar cells enable better light absorption and energy conversion, making renewable energy systems more efficient and viable.
7. How do nanomaterials contribute to advancements in medical applications, such as drug delivery systems?
Answer: Nanomaterials are designed to deliver drugs directly to target cells, improving treatment efficacy and minimizing side effects. Their small size allows them to navigate biological barriers, while surface modifications enable precise targeting. For example, nanoparticles loaded with therapeutic agents can release drugs in response to specific stimuli, such as pH changes in tumors.
8. How can nanotechnology revolutionize the design and performance of catalysts in chemical engineering?
Answer: Nanotechnology enables the creation of catalysts with controlled shapes, sizes, and compositions, optimizing their activity and selectivity. Nanocatalysts provide more active sites due to their high surface area, which accelerates reaction rates and reduces energy consumption. Innovations like bimetallic nanoparticles further enhance catalytic properties, enabling more efficient industrial processes.
9. What role does computational modeling play in the development of nanotechnology for chemical engineering?
Answer: Computational models simulate the behavior of nanomaterials, predicting their properties and interactions under various conditions. This accelerates the design process, reduces experimental costs, and helps identify optimal material configurations. For example, simulations can predict how nanoparticle size and shape influence catalytic efficiency, guiding experimental synthesis.
10. How does the incorporation of nanotechnology in chemical engineering impact global industries, such as energy, healthcare, and manufacturing?
Answer: In the energy sector, nanotechnology improves solar cells and batteries, advancing renewable energy adoption. In healthcare, it enables precise drug delivery and advanced imaging techniques. In manufacturing, it enhances materials like lightweight composites for transportation. These advancements drive efficiency, innovation, and sustainability across industries, creating economic and societal benefits.
11. What ethical considerations arise from the widespread adoption of nanotechnology in chemical engineering?
Answer: Ethical concerns include the potential for unequal access to nanotechnology benefits, unintended environmental impacts, and health risks. Addressing these requires transparent communication, inclusive policy development, and investment in safety research. Ensuring equitable access and minimizing risks are essential for responsible innovation.
12. What future trends and breakthroughs can be anticipated in the field of nanotechnology within chemical engineering?
Answer: Future trends include self-assembling nanomaterials, nanosensors for real-time monitoring, and nanostructures for carbon capture. Breakthroughs in nanomanufacturing techniques will enable large-scale production with lower costs. Advancements in machine learning and AI will further optimize nanomaterial design, driving innovations in energy, healthcare, and environmental applications.
