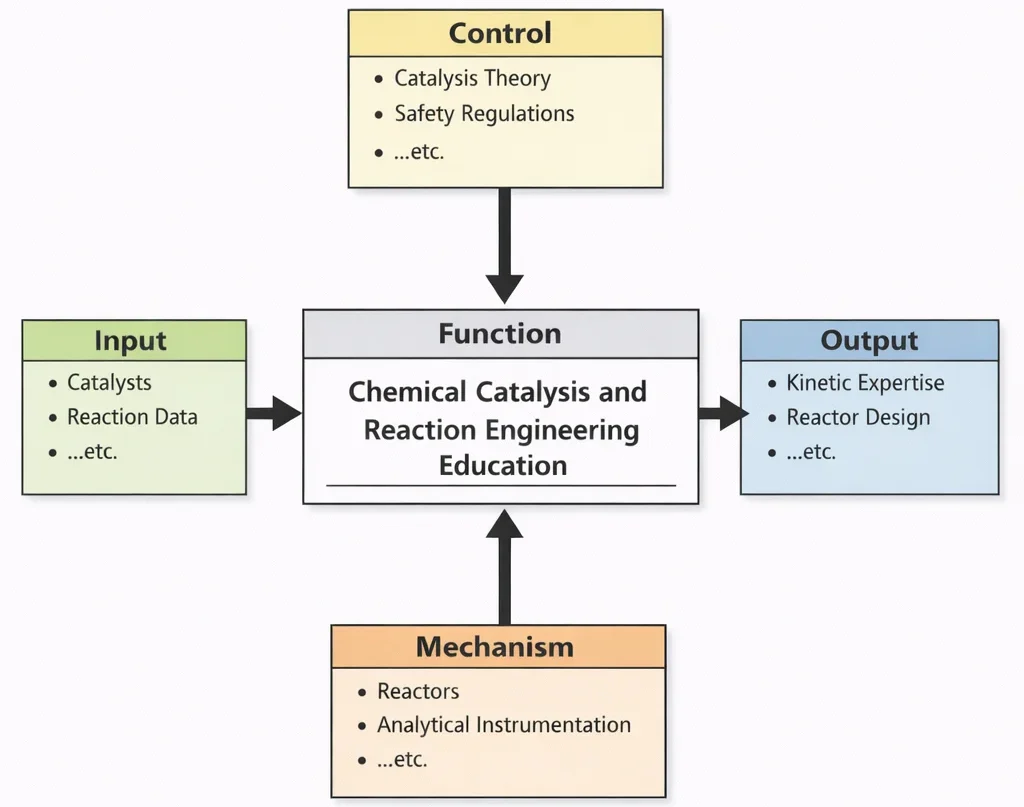
Chemical Catalysis and Reaction Engineering Education, viewed through this diagram, teaches students how to move from “a reaction that happens” to “a reaction we can control.” The Inputs anchor learning in reality: the catalyst itself (its surface, structure, and stability) and the reaction data that reveal what actually occurs under different conditions. The Controls provide the rules of interpretation and the rules of responsibility—catalysis theory explains mechanisms and rate behavior, while safety regulations prevent hazardous shortcuts and enforce disciplined operating boundaries. The Mechanisms are where knowledge becomes skill: students work with reactors and analytical tools to generate trustworthy data, fit kinetic models, identify bottlenecks such as mass transfer or deactivation, and test improvements. The Outputs are engineers who can predict and shape reaction behavior, and who can design reactors and operating strategies that deliver desired conversion and selectivity reliably—from the lab bench to industrial scale.
Chemical Catalysis and Reaction Engineering form the heart of modern chemical process industries, where transformation of raw materials into valuable products occurs with optimal efficiency and control. Students entering this field must build a firm foundation in Chemical Engineering, as it offers the basic principles of mass, heat, and momentum transfer essential to reactor design. Closely related areas such as Biochemical Engineering explore catalytic reactions driven by enzymes and microorganisms, blending biology with classical reaction engineering.
The ability to manage energy efficiently is essential in catalytic systems, linking this field with Chemical Energy Systems Engineering. The nature and durability of catalysts also demand expertise in Chemical Materials Engineering, where high-temperature behavior, surface area, and stability of materials are critical. To implement industrial-scale transformations, students need to master Chemical Process Engineering, which integrates reactors into plant-wide systems.
Modern chemical engineers increasingly rely on simulations and digital optimization, making Computational Chemical Engineering an indispensable tool for analyzing kinetics, modeling heat release, and minimizing by-products. Real-world applications extend to sectors like Food and Beverage Engineering, where enzymes or heterogeneous catalysts accelerate safe and consistent food processing. Research on surface reactions at the nano-level is advancing rapidly under Nanotechnology in Chemical Engineering.
Innovations in reactor lining, catalyst carriers, and polymers require knowledge of Polymer and Plastics Engineering. Large-scale chemical production facilities must work in harmony with structural elements planned by Civil Engineering experts, while execution logistics are typically coordinated through Construction Management. The safety of high-temperature or high-pressure catalytic operations also intersects with the knowledge found in Earthquake and Disaster Engineering.
Infrastructure planning for reactors must consider soil behavior, requiring collaboration with Geotechnical Engineering. Industrial reactor buildings are designed with principles from Structural Engineering to withstand mechanical loads and thermal expansion. Distribution and delivery of raw materials or products call on expertise in Transportation Engineering and broader layout designs informed by Urban and Regional Planning.
Water is a critical medium in many catalytic processes, whether as solvent or coolant, making Water Resources Engineering relevant for sustainability and environmental compliance. Electrical infrastructure for sensors, heaters, and mixers ties in closely with Electrical and Electronic Engineering. Emerging monitoring technologies from Biomedical Electronics can also be adapted for chemical safety systems.
Industrial communication protocols used in plant-wide process control stem from Communication Engineering. Control algorithms ensure that reactors operate under optimal temperature and concentration conditions, supported by Control Systems Engineering. Engineers deploy microcontrollers, actuators, and safety switches designed in Electronics Engineering and Embedded Systems and Microelectronics. Finally, the precision required in tracking reaction progress and maintaining compliance with regulatory bodies is made possible through Instrumentation and Measurement, cementing its importance in this discipline.

This image visualises the heart of catalysis and reaction engineering: guiding chemical change with precision. At the centre is a reactor-like apparatus rendered with a luminous, schematic glow, suggesting the controlled environment where reactions occur—temperature, pressure, mixing, and flow carefully managed. The scientist’s tablet and the floating molecular diagrams (rings, bonds, and symbolic formulas) represent the way reaction engineers connect theory to practice: using kinetics, mechanisms, and real-time measurements to understand how molecules transform and how catalysts speed up desired pathways while suppressing unwanted side reactions. The data-style overlays hint at key concerns such as conversion, selectivity, yield, heat release, mass transfer, and reactor safety. Overall, the scene communicates how catalysts and well-designed reactors make industrial chemistry cleaner, faster, and more economical—supporting the production of fuels, polymers, pharmaceuticals, and sustainable chemicals.
Chemical Catalysis and Reaction Engineering – Invisible FAQ
- How does chemical catalysis relate to overall chemical reaction engineering?
- Chemical catalysis provides the molecular-level mechanisms and active sites that control how fast and how selectively a reaction proceeds, while reaction engineering uses kinetics, thermodynamics and transport phenomena to design reactors and operating conditions that exploit those catalytic properties at laboratory, pilot and industrial scale.
- Why are activation energy and reaction pathways important for catalyst design?
- Catalysts work by offering an alternative reaction pathway with a lower activation energy. Understanding this pathway helps engineers tune active sites, supports and promoters so that the catalyst accelerates the desired route while suppressing side reactions, improving selectivity and energy efficiency.
- What is the difference between intrinsic kinetics and mass-transfer-limited rates?
- Intrinsic kinetics describe the rate of the surface reaction itself, assuming reactants reach active sites without limitation. In practice, external film resistance and internal pore diffusion can slow the overall rate. Reaction engineers must distinguish between these regimes to decide whether to modify the catalyst, the reactor hydrodynamics or both.
- How do homogeneous and heterogeneous catalysts compare in terms of process design?
- Homogeneous catalysts often give excellent selectivity and well-defined mechanisms but can be difficult to separate and recycle, influencing downstream design. Heterogeneous catalysts are easier to separate and reuse, but require careful management of contact time, surface area and flow patterns to overcome diffusion limitations and deactivation.
- Why is reactor selection (CSTR, PFR, packed bed, fluidised bed) critical in catalytic processes?
- Different reactor types produce different residence time distributions, mixing characteristics and heat- and mass-transfer behaviour. For catalytic systems, these differences strongly affect conversion, selectivity, hot-spot formation and catalyst life, so reactor choice must be aligned with both the kinetics and the heat effects of the reaction.
- What are the main mechanisms of catalyst deactivation in industrial plants?
- Common deactivation mechanisms include sintering at high temperatures, poisoning by trace impurities, coking or fouling by heavy by-products, and structural changes in the support. Understanding these mechanisms allows engineers to adjust feed purification, operating windows and regeneration strategies to extend catalyst life.
- How is computational modelling used in catalysis and reaction engineering?
- Computational tools range from molecular modelling and density functional theory (DFT) for active site design to reactor-scale CFD coupled with kinetic models. These simulations support scale-up, identify hot spots, quantify diffusion effects and test operating strategies before expensive pilot or plant trials are undertaken.
- What safety issues are particularly important in catalytic reactors?
- Exothermic catalytic reactions can create hot spots, pressure excursions and runaway risks if heat removal or feed composition are not carefully managed. Reaction engineers incorporate relief systems, robust control strategies, safe start-up and shutdown procedures, and inherently safer design choices to reduce these hazards.
- How does catalysis contribute to cleaner and more sustainable processes?
- Catalysts enable lower-temperature, lower-pressure operation, improved atom economy and higher selectivity toward desired products. This reduces energy consumption, greenhouse gas emissions and waste formation, supporting green chemistry principles and more sustainable industrial production.
- What skills are important for students interested in catalysis and reaction engineering?
- Students benefit from a strong foundation in physical chemistry, thermodynamics, reaction kinetics, transport phenomena, numerical methods and process safety. Familiarity with laboratory characterisation techniques and process simulation software also helps bridge molecular-level understanding and reactor-scale design.
- Chemical Engineering topics:
- Chemical Engineering – Overview
- Chemical Process Engineering
- Chemical Catalysis & Reaction Engineering
- Chemical Materials Engineering
- Chemical Energy Systems Engineering
- Polymer & Plastics Engineering
- Nanotechnology in Chemical Engineering
- Biochemical Engineering
- Food & Beverage Engineering
- Computational Chemical Engineering
Table of Contents
Core Concepts in Chemical Catalysis and Reaction Engineering
Catalysis
- Definition:
The process of increasing the rate of a chemical reaction by introducing a catalyst. Catalysts work by lowering the activation energy required for a reaction, allowing it to proceed more quickly and efficiently. - Types of Catalysis:
- Homogeneous Catalysis: Catalysts and reactants are in the same phase (typically liquid).
- Heterogeneous Catalysis: Catalysts and reactants are in different phases (usually solid catalyst with gaseous or liquid reactants).
- Enzymatic (Biocatalysis): Biological catalysts (enzymes) are used for reactions under mild conditions.
- Catalyst Properties:
- Activity: Ability to accelerate reaction rates.
- Selectivity: Ability to direct the reaction toward a desired product.
- Stability: Resistance to deactivation over time.
Reaction Engineering
- Definition:
The study and design of chemical reactors where chemical reactions occur. It involves analyzing reaction kinetics, thermodynamics, and transport processes to optimize production efficiency. - Key Aspects:
- Reaction Kinetics: Study of reaction rates and mechanisms.
- Thermodynamics: Understanding energy changes and equilibrium.
- Mass and Heat Transfer: Ensuring efficient distribution of heat and reactants.
- Types of Reactors:
- Batch Reactors: Used for small-scale, high-value reactions.
- Continuous Stirred-Tank Reactors (CSTR): Well-mixed continuous processes.
- Plug Flow Reactors (PFR): High-efficiency continuous reactors for large-scale production.
- Packed Bed Reactors: Common in catalytic processes with solid catalysts.
Reaction Kinetics and Mechanisms
- Reaction Rate Laws:
Mathematical expressions relating the rate of a reaction to the concentration of reactants. - Catalytic Mechanisms:
- Adsorption: Reactants bind to the catalyst surface.
- Reaction: Transformation of adsorbed species into products.
- Desorption: Products detach from the catalyst surface.
Catalyst Design and Optimization
- Active Sites:
Specific regions on the catalyst where reactions occur. - Support Materials:
Porous materials (e.g., alumina, silica) that disperse the active phase. - Promoters and Inhibitors:
Additives that enhance or suppress catalytic activity.
Process Integration and Scale-Up
- Lab-to-Plant Transition:
Scaling up catalytic reactions from laboratory studies to industrial production. - Process Intensification:
Improving efficiency through novel reactor designs and heat integration.
Key Applications of Chemical Catalysis and Reaction Engineering
Petrochemical and Refining Industry
- Catalytic Cracking:
Breaking down large hydrocarbon molecules into lighter fuels (e.g., gasoline, diesel) using zeolite catalysts. - Hydrotreating:
Removing sulfur and nitrogen compounds from fuels to meet environmental regulations. - Catalytic Reforming:
Converting low-octane hydrocarbons into high-octane gasoline components.
Chemical and Polymer Production
- Ammonia Synthesis (Haber-Bosch Process):
Iron-based catalysts facilitate the production of ammonia for fertilizers. - Ethylene Oxide Production:
Silver catalysts oxidize ethylene to create a precursor for plastics and antifreeze. - Polymerization Catalysts:
Ziegler-Natta and metallocene catalysts for producing polyethylene and polypropylene.
Environmental and Emission Control
- Automotive Catalytic Converters:
Platinum, palladium, and rhodium catalysts convert toxic gases (CO, NOₓ, hydrocarbons) into harmless emissions (CO₂, N₂, H₂O). - Selective Catalytic Reduction (SCR):
Converts nitrogen oxides (NOₓ) into nitrogen and water using ammonia and catalysts. - Carbon Capture and Utilization (CCU):
Catalytic processes for converting captured CO₂ into fuels and chemicals.
Renewable Energy and Sustainable Processes
- Hydrogen Production:
- Steam Methane Reforming (SMR): Uses nickel-based catalysts to produce hydrogen.
- Water Splitting: Photocatalysts and electrocatalysts split water into hydrogen and oxygen.
- Fuel Cells:
Platinum-based catalysts drive electrochemical reactions to generate electricity. - Biomass Conversion:
Catalytic pyrolysis and hydroprocessing turn biomass into biofuels.
Fine Chemicals and Pharmaceuticals
- Asymmetric Catalysis:
Producing chiral drugs with high purity using specialized catalysts. - Green Chemistry:
Catalytic reactions that minimize waste and energy consumption.
Emerging Technologies in Chemical Catalysis and Reaction Engineering
Nanocatalysis
- Definition: Nanocatalysis involves the use of nanoscale catalysts that possess high surface area-to-volume ratios, enhancing reaction rates and selectivity. These materials enable greater efficiency in chemical transformations due to quantum and surface effects unique to the nanoscale.
- Materials Used: Metal nanoparticles (e.g., Pt, Au, Pd), metal oxides, and carbon-based nanomaterials such as graphene.
- Applications:
- Hydrogen production via water splitting and reforming reactions.
- Emission control in automotive catalytic converters.
- Selective oxidation in the fine chemicals industry.
- Learn more about nanocatalysis
Photocatalysis
- Definition: Photocatalysis leverages light to activate catalysts, enabling chemical reactions to occur with minimal external energy input. It is a sustainable approach for driving redox reactions using solar or artificial light sources.
- Mechanism: Absorbed light energy excites electrons, generating electron-hole pairs that initiate redox reactions with adsorbed molecules.
- Applications:
- Degradation of pollutants in water and air purification systems.
- Photocatalytic splitting of water for clean hydrogen production.
- CO₂ conversion into value-added products using sunlight.
Electrocatalysis
- Definition: Electrocatalysis involves the facilitation of electrochemical reactions at electrode surfaces, crucial for energy technologies like fuel cells, batteries, and electrolyzers.
- Key Reactions: Oxygen reduction reaction (ORR), hydrogen evolution reaction (HER), oxygen evolution reaction (OER), and CO₂ electroreduction.
- Applications:
- Electrolytic water splitting for hydrogen generation.
- Carbon capture and conversion to alcohols or hydrocarbons.
- Rechargeable lithium-air and zinc-air batteries.
Single-Atom Catalysts (SACs)
- Definition: SACs consist of isolated metal atoms dispersed on supports like graphene or metal oxides. They exhibit maximum atom efficiency, high catalytic activity, and unique selectivity.
- Advantages: Ultra-high dispersion of metal atoms, minimal waste, and tailored active sites for specific reactions.
- Applications:
- Low-temperature CO oxidation and NOx reduction in emission control.
- Hydrogenation reactions in pharmaceutical synthesis.
- Electrochemical CO₂ reduction and N₂ fixation.
Artificial Intelligence (AI) in Catalyst Design
- Overview: AI and machine learning (ML) algorithms are being used to model catalytic systems, screen materials, and predict reaction pathways. These tools accelerate discovery cycles and reduce experimental costs.
- Methods: Deep learning for pattern recognition in reaction kinetics, generative models for designing novel catalysts, and reinforcement learning for optimizing experimental protocols.
- Applications:
- Predicting catalyst activity, stability, and selectivity with ML models.
- Automating laboratory synthesis and testing via robotic systems guided by AI.
- Designing reaction networks for biomass conversion and sustainable chemistry.
- AI for catalyst discovery in chemical catalysis
Challenges in Chemical Catalysis and Reaction Engineering
Catalyst Deactivation:
- Overview: One of the primary challenges in catalysis is the gradual loss of catalytic performance due to mechanisms such as sintering, poisoning, fouling, and coking. These issues reduce efficiency, increase costs, and require frequent replacement or regeneration of catalysts.
- Types of Deactivation:
- Sintering: Catalyst particles merge under high temperatures, reducing active surface area.
- Poisoning: Impurities like sulfur or chlorine bind irreversibly to active sites, rendering them inactive.
- Coking: Carbon deposits block active areas in hydrocarbon processing.
- Fouling: Physical obstruction by feed impurities or deposition layers.
- Mitigation Strategies: Use of more thermally stable catalysts, improved reactor design, protective coatings, and periodic regeneration processes.
Selectivity Control:
- Challenge: Ensuring that the catalyst produces primarily the desired product while minimizing the formation of by-products is essential for efficiency and cost reduction.
- Factors Influencing Selectivity: Reaction temperature, pressure, reactant concentration, and catalyst morphology all influence outcomes.
- Solutions: Precision engineering of active sites and the use of promoters or inhibitors to fine-tune reaction pathways.
Scale-Up and Process Integration:
- Lab-to-Industry Gap: Catalytic processes that perform well on a small scale may encounter technical challenges when scaled up for commercial use.
- Common Barriers: Nonlinear heat and mass transfer, flow dynamics, catalyst uniformity, and process control complexity.
- Best Practices: Simulation tools, pilot testing, and advanced scale-up methodologies like digital twin modeling are increasingly used to bridge this gap.
Cost of Precious Metals:
- Problem: Many high-performance catalysts rely on expensive and rare metals like platinum, rhodium, and palladium, which increases the overall cost of the process.
- Solutions: Development of non-precious metal alternatives, alloy catalysts, and atom-efficient single-atom catalysts.
- Explore innovations in catalyst materials that aim to reduce dependency on costly elements.
Environmental Impact:
- Challenge: Traditional catalytic processes can consume significant energy and produce harmful by-products, which pose environmental risks.
- Green Chemistry Approaches: Researchers are developing more sustainable catalytic processes that operate under milder conditions, use renewable feedstocks, and minimize waste.
- Frameworks: Implementation of Life Cycle Assessment (LCA), circular economy principles, and green solvent systems help reduce the environmental footprint of catalytic technologies.
Future Directions in Chemical Catalysis and Reaction Engineering
Sustainable Catalysis:
- Overview: The push toward environmental responsibility is driving the development of sustainable catalytic processes. These aim to minimize waste, reduce reliance on non-renewable resources, and promote eco-friendly practices.
- Recyclable Catalysts: Catalysts designed for repeated use without loss of activity, such as supported metal nanoparticles or magnetic nanocatalysts, are being studied for industrial deployment.
- Bio-Based Catalysts: Enzyme catalysts and biogenic materials derived from natural sources are being used in low-energy transformations. These are especially relevant for pharmaceutical and food industries.
- Example: The use of biochar-supported metal catalysts in biomass conversion offers a renewable platform for green chemistry applications.
- Learn more about sustainable catalysis in green chemistry.
Carbon Neutral Processes:
- Goal: With climate change a pressing issue, catalysis is being explored as a critical tool for achieving carbon neutrality through carbon capture, storage, and utilization (CCUS) strategies.
- Catalytic CO2 Conversion: New catalysts are being designed to convert CO2 into valuable products like methanol, hydrocarbons, and polymers under mild conditions.
- Integration with Industrial Processes: Incorporating these systems into power plants, cement factories, and steel industries offers a path to decarbonize heavy industry.
- Electrocatalytic Approaches: Use of renewable electricity to drive the reduction of CO2 into chemical fuels.
Integration with Renewable Energy:
- Hydrogen Production: Catalysts are essential for water electrolysis, a process that can generate hydrogen using solar or wind power. This green hydrogen serves as a clean energy carrier and feedstock.
- Power-to-X Technologies: Converting excess renewable energy into fuels and chemicals via catalytic reactions (e.g., ammonia, methane) is gaining traction as a storage strategy.
- Photo- and Electrocatalysis: These emerging fields allow direct use of sunlight and electricity to activate reactions, bypassing fossil energy inputs.
- Explore innovations in renewable hydrogen production.
Advanced Reactor Designs:
- Microreactors: These compact, highly efficient systems offer enhanced heat and mass transfer, ideal for fast and exothermic catalytic reactions.
- Modular Reactors: Portable and scalable reactor units enable distributed manufacturing and flexible deployment in remote areas.
- 3D-Printed Reactors: Additive manufacturing allows custom reactor geometries, tailored flow dynamics, and integration of sensing elements.
- Benefits: These designs lead to lower energy consumption, safer operation, and more precise control over reaction conditions.
Circular Economy Catalysis:
- Concept: Applying catalysis to recover and regenerate materials from waste, aligning chemical production with circular economy goals.
- Plastic Depolymerization: Catalysts enable the breakdown of complex polymers like PET and polystyrene into monomers for reuse in new materials.
- Waste-to-Chemicals: Organic and industrial waste streams can be chemically transformed into fuels, lubricants, or specialty chemicals using catalytic processes.
- Upcycling: Value-added conversion of waste materials into higher-grade products via selective catalysis.
Why Study Chemical Catalysis and Reaction Engineering
Understanding Chemical Reactions
This field examines how reactions occur and how to control them efficiently. Students study kinetics, thermodynamics, and reactor design. This knowledge is vital for developing industrial-scale chemical processes.
Catalyst Design and Mechanism
Students learn how catalysts accelerate reactions and influence selectivity. They explore material properties, surface science, and catalyst deactivation. Designing effective catalysts improves yields and reduces costs.
Reactor Engineering and Scale-Up
The course covers different reactor types and scale-up considerations. Students analyze residence time, mixing, and heat transfer. These skills support safe and efficient chemical production.
Applications in Green Chemistry
Catalysis enables cleaner and more sustainable chemical reactions. Students explore how to reduce waste and use renewable feedstocks. This aligns chemical engineering with environmental goals.
Industrial and Research Opportunities
Reaction engineering is critical in petrochemicals, pharmaceuticals, and materials science. Students can pursue roles in process development, R&D, or plant operations. The field is central to industrial innovation and efficiency.
Chemical Catalysis and Reaction Engineering: Conclusion
Chemical Catalysis and Reaction Engineering serve as foundational pillars of modern chemical innovation and manufacturing. This dynamic field plays a crucial role in optimizing reaction pathways, increasing yields, reducing energy consumption, and promoting the shift toward environmentally conscious industrial processes. With catalysts acting as molecular facilitators, complex chemical transformations that would otherwise require extreme conditions can now occur under milder, more sustainable circumstances.
Driving Efficiency Across Industries
Catalysis enhances the efficiency of countless processes across sectors—from refining and petrochemicals to pharmaceuticals and food processing. Advances in reaction engineering, such as the development of fluidized bed reactors and membrane-assisted systems, allow for precise control over reaction parameters, ensuring maximum output and minimal resource input. These breakthroughs reduce operational costs and contribute to the broader goals of energy conservation.
Key to Clean Energy and Green Chemistry
The global pursuit of decarbonization and clean energy relies heavily on innovations in catalysis. Electrocatalysts and photocatalysts enable the conversion of water and carbon dioxide into valuable fuels using renewable electricity, such as in hydrogen fuel production and artificial photosynthesis. As the world transitions away from fossil fuels, these technologies are becoming increasingly vital. Read more about the role of hydrogen catalysis in clean energy.
Enabling a Circular Economy
Catalysis is essential to the development of circular chemical processes. Techniques like catalytic depolymerization of plastics and waste-to-fuel conversion offer pathways to reduce landfill use and recover valuable resources. Reaction engineering innovations further amplify these effects by enabling continuous processes that reduce emissions and improve process intensification. For an overview of circular catalysis efforts, visit Nature Catalysis on circular economy chemistry.
Future Outlook: Smarter, Greener Systems
With the integration of artificial intelligence, machine learning, and IoT into catalytic research and reactor systems, the future of chemical manufacturing is becoming smarter and more autonomous. These digital enhancements support real-time monitoring, predictive maintenance, and optimization of reactions at scale. As we face mounting climate and resource challenges, the synergy of traditional catalysis with emerging technologies is expected to redefine industrial chemistry in the 21st century.
Ultimately, the convergence of sustainable catalyst development, advanced reactor design, and intelligent control systems underscores the transformative potential of Chemical Catalysis and Reaction Engineering. By enabling cleaner, faster, and more selective chemical processes, this field not only supports industrial innovation but also contributes decisively to global sustainability and resilience.
Chemical Catalysis and Reaction Engineering – Frequently Asked Questions (FAQ)
1. Why do engineers invest so much effort in developing catalysts?
Catalysts allow reactions to run faster, at lower temperatures and with better selectivity toward useful products. This means plants can save energy, reduce waste and avoid costly separation of unwanted by-products, making processes both more profitable and more sustainable.
2. What is the practical difference between homogeneous and heterogeneous catalysts?
Homogeneous catalysts share the same phase as the reactants, often giving very uniform reaction conditions and high selectivity, but they can be difficult to separate and recycle. Heterogeneous catalysts are usually solids in contact with gases or liquids, which simplifies separation and reuse but requires careful attention to surface area, diffusion and contact time.
3. How do catalyst structure and surface area affect performance?
The number, type and arrangement of active sites on a catalyst surface determine how molecules adsorb and react. High surface area and well-dispersed active sites maximise contact between reactants and catalyst, while tailored pore structures and promoters can steer reactions toward desired products and away from undesired side reactions.
4. What does a reaction engineer actually do in a real plant?
A reaction engineer links lab-scale kinetics and catalyst data to full-scale reactor and process design. They select reactor types, specify operating conditions, design heat removal and mixing strategies, troubleshoot performance issues, and work with other specialists to ensure that the reaction section is safe, efficient and reliable.
5. How do I decide whether to use a CSTR, PFR or packed-bed reactor?
The choice depends on reaction order, heat effects, desired conversion, and whether a catalyst is present. CSTRs are good for strong heat effects and thorough mixing, PFRs for high conversions and plug-flow behaviour, and packed-bed reactors for heterogeneous catalysts where contact time and pressure drop must be balanced.
6. Why is mass transfer such a concern in catalytic systems?
Even if the surface reaction is fast, reactants must reach the catalyst surface and products must diffuse away. If diffusion is slow, it can “choke” the reaction and hide the true kinetics. Designing particles, supports and reactor flows that minimise diffusion limitations is essential to get the most from a catalyst.
7. What causes catalysts to lose activity over time?
Catalysts can deactivate through sintering at high temperatures, poisoning by impurities such as sulfur, coking by heavy carbon deposits or structural changes in the support. Monitoring these mechanisms and planning for regeneration or replacement is a key part of long-term plant operation.
8. How does understanding reaction kinetics help with process optimisation?
Kinetic models show how rate depends on temperature, pressure and composition. This allows engineers to identify operating windows that maximise conversion and selectivity, design reactors of appropriate size, and predict how changes in feed or conditions will affect plant performance before changes are implemented.
9. Where does computational modelling fit into catalysis and reaction engineering?
Modelling tools help test reactor designs, explore temperature and concentration profiles, and evaluate different operating strategies without running expensive or risky experiments. At smaller scales, molecular simulations can guide catalyst formulation by predicting how different surfaces interact with reactants and intermediates.
10. How does this topic connect to sustainability and green chemistry?
Catalysis and reaction engineering support green chemistry by improving atom economy, reducing energy use, and lowering emissions and waste. Well-designed catalytic processes enable cleaner production of fuels, chemicals and materials, and play a central role in technologies such as emissions control, greener solvents and CO2 utilisation.
Chemical Catalysis and Reaction Engineering: Review Questions and Answers
-
What is chemical catalysis, and why is it so important in modern industry?
Answer: Chemical catalysis is the acceleration of a reaction by a substance—the catalyst—that participates in the reaction pathway but is regenerated at the end of the cycle. By lowering the activation energy and steering reaction pathways, catalysts allow processes to run faster, at milder conditions, and with higher selectivity toward desired products. In modern industry this translates into lower energy consumption, fewer by-products, smaller equipment and more sustainable production of fuels, chemicals and materials. -
How do homogeneous and heterogeneous catalysis differ in practice?
Answer: In homogeneous catalysis, the catalyst and reactants share the same phase, typically a liquid solution, which enables intimate molecular mixing and often excellent selectivity and controllable mechanisms. However, separating and recycling the catalyst can be challenging. In heterogeneous catalysis, the catalyst is usually a solid while reactants are gases or liquids. This makes separation and reuse simpler and is well-suited to fixed-bed or fluidised-bed reactors, but requires careful design of surface area, pore structure and flow patterns to overcome diffusion limitations and maintain high activity. -
How does catalyst design influence activity and selectivity of a reaction?
Answer: Catalyst design aims to create surfaces with the right number, type and environment of active sites. High surface area supports expose more active sites, while controlled pore structures manage access of molecules to those sites. Adjusting the electronic properties of metals or oxides, adding promoters and tailoring the support can change how strongly reactants, intermediates and products adsorb. These changes alter preferred pathways, so that the catalyst not only speeds up the reaction but also favours the formation of specific target products while suppressing unwanted side reactions. -
What is reaction engineering, and how does it connect with catalysis in real processes?
Answer: Reaction engineering is the discipline that uses kinetics, thermodynamics and transport phenomena to design and analyse reactors and reaction systems. When combined with catalysis, it connects molecular-scale behaviour at active sites with reactor-scale performance. Reaction engineers use kinetic data and catalyst properties to choose reactor types, define operating conditions, manage heat and mass transfer, and ensure that industrial units achieve the desired conversion, selectivity, safety and reliability. -
Which key factors must be considered when designing a chemical reactor?
Answer: Important factors include the intrinsic reaction kinetics, heat generation or absorption, mass-transfer requirements, mixing behaviour and residence time distribution. Engineers must also account for pressure drop, catalyst loading or residence time, and the need for temperature control to avoid hot spots or thermal runaway. Practical considerations such as start-up and shutdown procedures, maintainability and integration with upstream and downstream units are also central to robust reactor design. -
How do fixed-bed and fluidised-bed catalytic reactors differ in operation and typical use?
Answer: Fixed-bed reactors contain a stationary packed bed of solid catalyst through which reactant fluids flow. They offer simple construction and are widely used for gas-phase hydrogenation, oxidation and other processes, but can suffer from temperature gradients and diffusion limitations. Fluidised-bed reactors suspend small catalyst particles in an upward flow of gas or liquid, improving mixing, heat transfer and temperature uniformity. They are suited to strongly exothermic reactions, large throughputs and applications such as fluid catalytic cracking or certain gas-phase polymerisations. -
Why is mass transfer a critical consideration in catalytic reaction engineering?
Answer: In heterogeneous catalysis, reactants must travel from the bulk fluid to the external catalyst surface and then diffuse into pores before reacting, while products must diffuse out again. If these steps are slow relative to the surface reaction, they become rate-limiting, reducing observed activity and sometimes altering apparent selectivity. Engineers mitigate mass-transfer limitations by adjusting flow regimes to increase turbulence, reducing catalyst particle size, tailoring pore structures and choosing reactor designs that promote effective contact between reactants and catalyst. -
How does knowledge of reaction kinetics support optimisation of industrial chemical processes?
Answer: Quantitative kinetic models describe how reaction rates depend on temperature, pressure and composition, and how different reactions compete. Using this information, engineers can identify operating windows that maximise conversion and selectivity while controlling by-product formation. Kinetic models underpin reactor sizing, scale-up from laboratory to plant, evaluation of alternative feedstocks, and systematic troubleshooting when plant performance deviates from expectations. -
What are common mechanisms of catalyst deactivation, and how can they be managed?
Answer: Catalysts can deactivate through sintering of active particles at high temperature, poisoning by trace impurities such as sulfur or heavy metals, coking or fouling by heavy carbon deposits, or structural changes in the support. Management strategies include purifying feeds, choosing operating conditions that minimise deactivation, periodically regenerating catalysts by controlled oxidation or reduction steps, and designing materials that are intrinsically more resistant to the relevant deactivation mechanisms. -
How is computational modelling used in the development of catalytic processes and reactor design?
Answer: Computational modelling combines kinetic expressions with mass and energy balances, and often fluid dynamics, to simulate how catalytic reactors will behave under different conditions and scales. These models help evaluate alternative reactor concepts, predict temperature and concentration profiles, estimate hot-spot risks, and test process-control strategies before building pilot or full-scale units. This reduces experimental effort, speeds up development and deepens understanding of complex reaction–transport interactions.
Thought-Provoking Questions with Detailed and Elaborate Answers on Chemical Catalysis and Reaction Engineering
Why are catalysts not consumed in a reaction, and how does this property make them indispensable in chemical processes?
- Answer: Catalysts facilitate chemical reactions by lowering the activation energy required for reactants to transform into products. They achieve this by providing an alternative reaction pathway with lower energy requirements. Since they do not chemically alter themselves in the process, they can repeatedly participate in reactions without being consumed. This property makes them highly efficient, allowing for continuous reactions and reduced operational costs in industrial processes.
What are the primary factors that influence the efficiency of a catalyst, and how can they be optimized?
- Answer: Catalyst efficiency is influenced by factors such as surface area, active site availability, and structural stability. Optimization involves increasing the catalyst’s surface area through nanostructuring, ensuring uniform distribution of active sites, and enhancing stability under operating conditions. Using promoters or modifying the electronic environment around the active sites can further enhance catalytic activity and selectivity.
How do homogeneous and heterogeneous catalysts differ in their applications, and what are the advantages and disadvantages of each?
- Answer: Homogeneous catalysts operate in the same phase as reactants, typically liquid, allowing for uniform interaction at the molecular level. They excel in selectivity but are challenging to separate and recycle. Heterogeneous catalysts exist in a different phase, often solid, and facilitate easier separation and reuse. However, they may encounter limitations in mass transfer and uniformity. The choice between the two depends on the specific reaction requirements and scalability.
What challenges arise when scaling up a catalytic process from the laboratory to an industrial scale, and how can these challenges be addressed?
- Answer: Challenges include maintaining reaction kinetics, heat and mass transfer efficiency, and catalyst stability at larger scales. Addressing these challenges involves using computational models to simulate scaling effects, designing reactors with optimized geometry, and conducting pilot-scale trials to refine operational parameters. Ensuring consistency in catalyst quality and performance across scales is also critical.
Why is reaction kinetics crucial for designing chemical reactors, and how can understanding reaction rates improve reactor performance?
- Answer: Reaction kinetics provides insights into the speed of chemical reactions and the factors affecting them, such as temperature, pressure, and reactant concentration. This knowledge is essential for designing reactors that maximize yield and efficiency. For example, understanding exothermic or endothermic reactions allows engineers to incorporate temperature control systems, preventing thermal runaway or inefficiencies.
How does the choice of reactor type impact the efficiency of catalytic reactions, and what factors determine the selection of a reactor?
- Answer: Reactor type impacts how reactants interact with the catalyst and the overall reaction efficiency. For example, fixed-bed reactors are ideal for stable processes with minimal fouling, while fluidized-bed reactors are preferred for processes requiring uniform temperature and high mass transfer rates. Factors like reaction kinetics, heat generation, product separation, and scalability influence reactor selection.
What role does surface chemistry play in heterogeneous catalysis, and how can it be tailored to improve catalyst performance?
- Answer: Surface chemistry determines how reactants adsorb, react, and desorb on a catalyst’s surface. Tailoring surface properties, such as roughness, porosity, and electronic configuration, enhances reaction rates and selectivity. For instance, doping a catalyst with specific metals can modify the electronic structure of active sites, influencing how reactants interact and reducing unwanted byproducts.
What are the environmental implications of using catalysts in chemical processes, and how can sustainable catalysis be achieved?
- Answer: Catalysts reduce energy consumption and byproduct formation, minimizing the environmental footprint of chemical processes. Sustainable catalysis can be achieved by developing catalysts from abundant, non-toxic materials, enhancing catalyst recyclability, and designing processes that use renewable feedstocks. These approaches align industrial practices with environmental sustainability goals.
How can reaction engineering contribute to the development of green chemistry principles in industrial applications?
- Answer: Reaction engineering supports green chemistry by designing processes that maximize atom economy, reduce hazardous waste, and utilize safer solvents. For example, engineers can optimize reaction conditions to eliminate unnecessary steps or intermediates, reducing energy and resource consumption. Incorporating renewable feedstocks and alternative energy sources like microwaves or solar energy further promotes green practices.
Why is catalyst deactivation a major challenge in industrial applications, and what strategies can be employed to mitigate it?
- Answer: Catalyst deactivation occurs due to fouling, poisoning, thermal degradation, or sintering. This reduces efficiency and increases operational costs. Strategies to mitigate deactivation include periodic regeneration, using more robust catalyst materials, and implementing pre-treatment steps to remove impurities from reactants. Engineering processes to operate within the catalyst’s stability range is also essential.
What is the significance of designing multi-functional catalysts, and how do they enhance reaction pathways?
- Answer: Multi-functional catalysts combine different active sites to facilitate sequential or simultaneous reactions, improving efficiency and selectivity. For example, in a tandem reaction, one active site may activate the reactant while another catalyzes the desired transformation. These catalysts reduce the need for separate reaction steps, saving energy and time.
How can advancements in artificial intelligence and machine learning accelerate innovations in catalysis and reaction engineering?
- Answer: AI and machine learning analyze large datasets to identify patterns, predict catalyst behavior, and optimize reaction conditions. These technologies enable faster screening of catalyst candidates, real-time process control, and predictive maintenance of reactors. By reducing trial-and-error experiments, AI accelerates innovation and enhances the efficiency of catalytic processes.
These thought-provoking questions aim to encourage critical thinking and exploration of the intricate concepts and applications in chemical catalysis and reaction engineering, fostering curiosity and innovation.
