At the heart of quantum mechanics lies a revolutionary concept: the wave function, a mathematical entity that encodes the probabilities of all possible outcomes of a quantum system. Unlike classical physics, where objects have definite positions and velocities, quantum systems are described by a ψ (psi) function, which provides the likelihood of finding a particle in a particular state. This probabilistic view reshapes our understanding of reality and becomes particularly powerful when combined with quantum superposition, where particles can exist in multiple states simultaneously.
The evolution of a wave function over time is governed by Schrödinger’s Equation, the fundamental equation of quantum dynamics. It plays a role analogous to Newton’s laws in classical physics, but instead of predicting a specific trajectory, it yields a probability distribution. This equation enables predictions in various fields, from nuclear physics and condensed matter physics to the behavior of elementary particles under fundamental forces. It even provides the analytical foundation for the study of quantum tunneling, where particles pass through potential barriers seemingly impossible to cross.
To grasp Schrödinger’s framework, one must first explore the basics of physics and delve into modern physics, particularly through the lens of atomic physics. Here, understanding the structure of the atom and the arrangement of electrons via quantum numbers and electron configuration provides critical background. The probabilistic interpretation of these electron distributions flows directly from solutions to Schrödinger’s Equation.
The wave function also interacts meaningfully with other core quantum concepts. For example, the Heisenberg’s uncertainty principle illustrates how wave-like behavior leads to inherent limitations in measuring position and momentum. Likewise, quantum entanglement reveals that wave functions can be shared between particles across vast distances, resulting in correlations that defy classical logic. These concepts collectively arise from the same mathematical underpinnings found in quantum field theory, which extends the wave function formalism to entire fields.
Applications of Schrödinger’s Equation and wave functions span diverse domains. In nuclear science, it describes phenomena such as nuclear fission, fusion, and other nuclear reactions. It also plays a pivotal role in describing the behavior of radioactive isotopes. In bosons and fermions, it helps explain how particles acquire and distribute energy. Even broader areas like relativity and statistical mechanics have been linked with quantum formulations using the wave function framework.
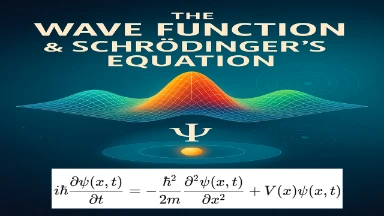
This visual representation captures the essence of quantum mechanics by depicting the interplay between the wave function and Schrödinger’s Equation. A glowing, probabilistic wave envelope flows across a warped spatial grid, symbolizing the spread and evolution of a quantum particle’s state. Floating around the wave are mathematical notations and elements of Schrödinger’s Equation, emphasizing its role in determining the system’s dynamics. The image conveys how the wave function encodes all possible outcomes and how observation collapses it into definite results. This foundational concept underpins predictions in atomic-scale physics, from electron orbitals to quantum tunneling and beyond.
Table of Contents
The Wave Function (Ψ): A Complete Description of a Quantum System
The wave function, denoted as Ψ (Psi), is the central mathematical tool in quantum mechanics. It provides the most complete description of the quantum state of a particle or system. Unlike classical mechanics, where a particle’s position and momentum can be precisely specified, quantum mechanics replaces these exact values with a probabilistic framework. The wave function encodes all the information about a particle’s physical state, including its position, momentum, energy, and other observable properties.
Mathematically, the wave function is a complex-valued function:
Where:
- r represents the position in space.
- t represents time.
The wave function can be complex, meaning it has both real and imaginary components:
Where a and b are real-valued functions, and i is the imaginary unit
Probability Interpretation: Born Rule
The physical meaning of the wave function was first proposed by Max Born in 1926. He suggested that the square of the wave function’s magnitude gives the probability density of finding a particle at a particular position in space at a given time.
Where:
- P(r,t) is the probability density.
- Ψ*(r,t) is the complex conjugate of the wave function.
- |Ψ(r,t)|² gives the likelihood of locating the particle in a small volume around r at time t.
This means the particle doesn’t have a definite position until measured; instead, it exists in a cloud of probabilities, often visualized as a “probability cloud” around a nucleus for electrons.
Schrödinger’s Equation: Governing the Evolution of the Wave Function
Proposed by Erwin Schrödinger in 1925, the Schrödinger Equation is the foundational equation of quantum mechanics. It describes how the wave function Ψ evolves over time and space, thereby determining the behavior of a quantum system.
Time-Dependent Schrödinger Equation (TDSE)
The most general form of Schrödinger’s equation accounts for systems where the wave function changes over time:
Where:
- i is the imaginary unit.
- ħ = h / 2π is the reduced Planck constant (ħ ≈ 1.0545718 × 10⁻³⁴ J·s).
- ∂/∂t represents the partial derivative with respect to time, describing how the wave function evolves over time.
- Ĥ is the Hamiltonian operator, representing the total energy of the system.
- Ψ(r,t) is the wave function, which encapsulates the quantum state of the system.
Hamiltonian Operator (Ĥ)
The Hamiltonian operator represents the total energy of a quantum system, including both kinetic and potential energy:
Where:
is the kinetic energy operator, with ∇2 being the Laplacian (a second-order spatial derivative).
- V(r) represents the potential energy as a function of position.
This form is particularly useful for systems like electrons in atoms, where the potential energy arises from electrostatic forces between charged particles.
Time-Independent Schrödinger Equation (TISE)
For systems where the potential energy does not change over time, Schrödinger’s equation simplifies to the time-independent form:
Where E is the total energy eigenvalue of the system. This equation is fundamental for solving problems related to the stationary states of quantum systems, such as electrons in atoms and particles in potential wells.
Physical Interpretation of Schrödinger’s Equation
The Schrödinger equation describes how quantum systems evolve. A few key implications are:
- Wave-Particle Duality: Particles behave as both waves and particles. The wave function describes their wave-like behavior, including interference and diffraction.
- Probability Waves: The wave function does not describe a particle’s physical shape but the probability of finding it in various locations.
- Quantum Superposition: A quantum system can exist in a combination of multiple states simultaneously until measured.
- Measurement and Collapse: Upon measurement, the wave function “collapses” to a specific state, and the probabilistic nature of quantum systems becomes an observable outcome.
Applications of the Schrödinger Equation
1. Atomic Structure
The Schrödinger equation accurately describes the behavior of electrons in atoms. Solutions for the hydrogen atom reveal discrete energy levels and explain the quantized spectra observed in atomic emissions and absorptions.
2. Quantum Chemistry
In molecules, the equation predicts chemical bonding, molecular orbitals, and reactions. It is essential for modeling how electrons are shared or transferred between atoms.
3. Quantum Tunneling
Schrödinger’s equation explains how particles can tunnel through energy barriers, critical in nuclear fusion, semiconductors, and quantum devices.
4. Quantum Computing
The manipulation of quantum states in qubits relies on the evolution described by Schrödinger’s equation, allowing superposition and entanglement to be harnessed for computation.
5. Nanotechnology
Understanding electron behavior in nanostructures requires solving the Schrödinger equation for complex potential wells and quantum dots.
Experimental Evidence Supporting the Wave Function
- Double-Slit Experiment: Particles such as electrons create interference patterns when not observed, behaving like waves. However, when observed, they act like particles, confirming the probabilistic interpretation of
- Electron Orbitals: The shapes of electron orbitals in atoms (s, p, d, f) are direct visualizations of the solutions to the Schrödinger equation.
- Quantum Tunneling Microscopy: The behavior of tunneling electrons in scanning tunneling microscopes is predicted by wave functions derived from Schrödinger’s equation.
Why Study The Wave Function and Schrödinger’s Equation
Describing Quantum States Mathematically
The wave function provides a complete mathematical description of a quantum system. Students learn that it contains all the information needed to calculate the probabilities of a particle’s measurable properties. This central concept represents the transition from classical to quantum thinking. Mastering it lays the groundwork for quantum analysis and prediction.
Solving Schrödinger’s Equation
Schrödinger’s equation governs the time evolution and spatial structure of quantum states. Students solve it for systems like potential wells, harmonic oscillators, and hydrogen atoms. These exercises develop skills in differential equations and boundary conditions. They build confidence in predicting quantum behavior.
Probability Densities and Measurement
The square of the wave function gives the probability density of finding a particle in a given location. Students interpret how measurements influence quantum systems through the collapse of the wave function. This helps clarify concepts like uncertainty and quantum expectation values. It supports a clear understanding of quantum measurement.
Visualizing Quantum States
Students use wave functions to visualize standing waves, energy levels, and tunneling phenomena. These visual tools enhance comprehension of abstract mathematical solutions. They help bridge intuition and formalism. They support learning across multiple representations of quantum behavior.
Foundations for Advanced Quantum Physics
Wave functions and Schrödinger’s equation underpin topics in quantum chemistry, atomic structure, and particle physics. Students use these tools in more advanced areas like spin, angular momentum, and perturbation theory. Understanding them is essential for all quantum fields. It prepares students for both academic research and applied science.
Conclusion
The wave function and Schrödinger’s equation are the foundation of quantum mechanics. The wave function encapsulates all possible information about a quantum system, and its probabilistic interpretation challenges classical ideas of determinism. Schrödinger’s equation dictates how this wave function evolves, predicting the behavior of particles in various physical contexts. Together, they provide a comprehensive and mathematically rigorous framework for understanding and predicting the counterintuitive behaviors of the quantum world. These concepts not only deepen our understanding of the universe but also drive technological advancements in electronics, energy, computing, and nanotechnology.
Frequently Asked Questions: The Wavefunction & Schrödinger’s Equation
1. What is the wavefunction in quantum mechanics?
In quantum mechanics, the wavefunction is a mathematical function that fully describes the quantum state of a system. Its complex value at each point in space encodes information about the probability amplitude of finding a particle in a particular position or state, and its squared magnitude gives the probability density of detecting the particle there.
2. Why is the wavefunction important?
The wavefunction is central to quantum mechanics because it encapsulates all the information about a particle or system’s state. It allows us to compute probabilities of outcomes for measurements, determine how the system evolves, and predict interference and other uniquely quantum phenomena.
3. What is the Schrödinger equation?
The Schrödinger equation is the fundamental equation of motion in non-relativistic quantum mechanics. It describes how the wavefunction of a quantum system evolves in time (time-dependent form) or determines stationary states and energy levels (time-independent form).
4. What does the time-independent Schrödinger equation tell us?
The time-independent Schrödinger equation provides the possible stationary states (eigenstates) of a quantum system and their associated energy levels. Solving it for a given potential yields wavefunctions that describe how the particle’s probability is distributed in space when it occupies a particular energy eigenstate.
5. How does the wavefunction evolve over time?
According to the time-dependent Schrödinger equation, the wavefunction evolves deterministically in time. The evolution preserves the total probability (normalisation) and encodes how amplitudes change and interfere, leading to dynamical quantum behaviour such as tunnelling, oscillations, and superposition changes.
6. What is probability density and how is it related to the wavefunction?
Probability density is the value given by the squared magnitude of the wavefunction at a point in space. It indicates the probability per unit volume of finding the particle near that point when a measurement of position is made. Integrating the probability density over a region gives the probability of locating the particle in that region.
7. Why must the wavefunction be normalised?
Normalization ensures that the total probability of finding the particle somewhere in space is 1. Without normalisation, the wavefunction would not yield valid probability estimates, making it physically meaningless. Normalisation is a fundamental requirement for any acceptable solution of the Schrödinger equation.
8. How are boundary conditions used when solving the Schrödinger equation?
Boundary conditions (such as the wavefunction going to zero at infinity, or being continuous at boundaries) are necessary to select physically meaningful solutions when solving the Schrödinger equation. They ensure the wavefunction is well-behaved, normalisable, and corresponds to allowed energy states.
9. How does the wavefunction explain interference and quantum phenomena?
Because the wavefunction is generally a complex quantity, different parts of it can interfere when they overlap. This interference leads to phenomena such as diffraction, quantum beats, tunnelling, and superposition behaviour that have no analogue in classical physics. The Schrödinger equation governs how these interference patterns evolve over time.
10. Is the wavefunction physically real or just a mathematical tool?
Whether the wavefunction is physically real or just a mathematical tool is a subject of interpretation in quantum mechanics. Some interpretations treat it as a real field that describes the physical state, while others view it as encoding our knowledge (or information) about the system. Regardless of interpretation, it provides accurate predictions for experiments.
11. Why should students learn about the wavefunction and Schrödinger equation before university?
Understanding the wavefunction and Schrödinger equation gives students a foundation for all of quantum mechanics. It connects the abstract formalism with concrete ideas like energy levels, probability, and wave behaviour, making it easier to grasp more advanced topics such as atomic structure, quantum states, tunnelling, and quantum computing.
12. What are some key quantum phenomena that arise from the wavefunction?
Key quantum phenomena arising from the wavefunction include quantised energy levels, interference patterns, tunnelling through barriers, superposition of states, and probabilistic measurement outcomes — all of which challenge classical intuition and illustrate the unique nature of quantum systems.
Review Questions and Answers:
1. What is the quantum wavefunction and what does it represent?
Answer: The wavefunction is a complex mathematical function that encodes all the information about a quantum system. Its squared modulus represents the probability density of finding a particle in a given region of space.
2. How is the wavefunction interpreted in quantum mechanics?
Answer: In quantum mechanics, the wavefunction is interpreted probabilistically. It provides the probability amplitude for different outcomes, meaning that measurement outcomes are inherently uncertain until observed.
3. What is Schrödinger’s Equation and why is it important?
Answer: Schrödinger’s Equation is a fundamental differential equation that governs the time evolution of a quantum system’s wavefunction. It is essential because it allows us to calculate how quantum states change over time and predict experimental outcomes.
4. How does the time-dependent Schrödinger Equation differ from the time-independent version?
Answer: The time-dependent Schrödinger Equation describes the evolution of the wavefunction over time, while the time-independent version is used for systems with stationary states, providing energy eigenvalues and corresponding eigenfunctions.
5. What are eigenstates and eigenvalues in the context of Schrödinger’s Equation?
Answer: Eigenstates are specific wavefunctions that remain unchanged (except for a phase factor) when an operator is applied, and their corresponding eigenvalues are the measurable quantities, such as energy levels, associated with these states.
6. What is meant by the normalization of the wavefunction?
Answer: Normalization ensures that the total probability of finding the particle somewhere in space is one. Mathematically, it requires that the integral of the wavefunction’s squared modulus over all space equals one.
7. How do potential energy functions influence solutions of Schrödinger’s Equation?
Answer: The potential energy function in Schrödinger’s Equation determines the shape of the wavefunction and the quantized energy levels of the system. Different potentials lead to different bound and scattering states.
8. What is the significance of the probability amplitude in the wavefunction?
Answer: The probability amplitude, given by the wavefunction’s complex value, contains both magnitude and phase information. Its squared modulus gives the probability density for measurements, while the phase affects interference and superposition phenomena.
9. How do operators act on the wavefunction in quantum mechanics?
Answer: Operators correspond to physical observables (like position, momentum, and energy) and, when acting on the wavefunction, yield eigenvalues that represent possible measurement outcomes, thereby linking abstract theory with observable quantities.
10. How has Schrödinger’s Equation advanced our understanding of quantum systems?
Answer: Schrödinger’s Equation has provided a powerful framework for predicting and explaining the behavior of atoms, molecules, and solid-state systems. It has led to the development of technologies such as lasers, semiconductors, and quantum computers.
Thought-Provoking Questions and Answers
1. How might the probabilistic nature of the wavefunction influence our understanding of reality?
Answer: The probabilistic interpretation of the wavefunction suggests that reality at the quantum level is not deterministic but governed by probabilities. This challenges the classical view of a well-defined reality and raises philosophical questions about observation, measurement, and the role of the observer in shaping outcomes.
2. What are the implications of wavefunction collapse for the concept of free will and determinism?
Answer: Wavefunction collapse implies that quantum systems do not have predetermined states until measured, suggesting that randomness plays a fundamental role. This challenges deterministic models of the universe and raises debates about the extent to which free will might be influenced by quantum indeterminacy.
3. How does the concept of superposition, as described by the wavefunction, impact the development of quantum computing?
Answer: Superposition allows quantum bits (qubits) to exist in multiple states simultaneously, enabling parallel computation and potentially exponential speed-ups for certain problems. This principle is the cornerstone of quantum computing, which promises to revolutionize data processing and problem solving.
4. Can the mathematical structure of the wavefunction provide insights into the nature of consciousness?
Answer: Some speculative theories propose that the superposition and entanglement inherent in the wavefunction might play a role in brain processes. While controversial, exploring these ideas could offer novel perspectives on consciousness and the intersection of quantum physics with neuroscience.
5. How might advancements in solving Schrödinger’s Equation lead to new materials with exotic properties?
Answer: Improved methods for solving Schrödinger’s Equation allow for accurate predictions of electronic structures and energy levels in materials. This can lead to the design of novel materials with tailored properties, such as superconductors or topological insulators, driving innovations in technology and energy.
6. What challenges exist in extending Schrödinger’s Equation to relativistic quantum systems, and how are they addressed?
Answer: Schrödinger’s Equation is non-relativistic, so it does not account for effects at speeds close to light. Relativistic quantum theories like the Dirac Equation are used instead. The challenge is to integrate relativity and quantum mechanics, an ongoing pursuit in the field of quantum field theory.
7. How does the phase information in the wavefunction affect interference patterns observed in experiments?
Answer: The phase of the wavefunction is crucial for interference; when two wavefunctions overlap, their phases determine whether they interfere constructively or destructively. This interference is observable in experiments like the double-slit experiment, highlighting the wave-like properties of particles.
8. In what ways might the concept of a “virtual” wavefunction influence our interpretation of quantum field theory?
Answer: In quantum field theory, virtual particles and fluctuating fields contribute to the observable properties of matter. The concept of a virtual wavefunction suggests that the vacuum itself is dynamic, influencing forces and interactions, which may lead to a deeper understanding of phenomena like the Casimir effect.
9. How can the uncertainty in the wavefunction’s parameters be minimized in experimental setups, and what are the limits of such minimization?
Answer: Techniques like laser cooling and magnetic trapping can reduce uncertainties by isolating and controlling quantum systems. However, the Heisenberg uncertainty principle sets a fundamental limit on how precisely position and momentum can be simultaneously known, ensuring that some uncertainty always remains.
10. What role does the normalization of the wavefunction play in ensuring the consistency of quantum theory?
Answer: Normalization guarantees that the total probability of finding a particle is one, ensuring that the mathematical predictions of quantum theory align with physical reality. It is a critical requirement for the internal consistency of the theory and for making meaningful predictions about measurement outcomes.
11. How might future breakthroughs in solving complex versions of Schrödinger’s Equation impact our understanding of chemical reactions?
Answer: Advanced computational techniques for solving Schrödinger’s Equation in complex systems could lead to more accurate models of chemical reactions, enabling the design of catalysts and new pharmaceuticals with greater efficiency and reduced environmental impact.
12. What philosophical questions does the non-deterministic evolution of the wavefunction raise about the nature of scientific predictions?
Answer: The non-deterministic evolution of the wavefunction, governed by probabilities rather than certainties, raises questions about the nature of prediction in science. It challenges the idea that the universe is fully knowable and forces us to consider whether scientific predictions are inherently statistical, reflecting a deeper, probabilistic structure of reality.
Numerical Problems and Solutions
1. Calculate the energy of a photon with a wavelength of 500 nm using E = hc/λ. (h = 4.1357×10⁻¹⁵ eV·s, c = 3.0×10⁸ m/s)
Solution:
λ = 500 nm = 500×10⁻⁹ m
E = (4.1357×10⁻¹⁵ eV·s × 3.0×10⁸ m/s) / (500×10⁻⁹ m)
≈ 1.2407×10⁻⁶ eV·m / 500×10⁻⁹ m
≈ 2.4814 eV.
2. Determine the ground state energy of an electron in a one-dimensional infinite potential well of width L = 1.0 nm using E₁ = h²/(8mL²). (m = 9.11×10⁻³¹ kg, h = 6.626×10⁻³⁴ J·s)
Solution:
L = 1.0×10⁻9 m
E₁ = (6.626×10⁻³⁴)² / (8 × 9.11×10⁻³¹ kg × (1.0×10⁻9 m)²)
= 4.39×10⁻67 J²·s² / (7.288×10⁻48 kg·m²)
≈ 6.02×10⁻20 J
Converting to eV: 6.02×10⁻20 J / 1.602×10⁻19 J/eV ≈ 0.376 eV.
3. Compute the de Broglie wavelength of an electron with kinetic energy 50 eV. (Use E = p²/(2m) and λ = h/p)
Solution:
E = 50 eV = 50 × 1.602×10⁻19 J = 8.01×10⁻18 J
p = √(2mE) = √(2 × 9.11×10⁻³¹ kg × 8.01×10⁻18 J)
≈ √(1.459×10⁻47) ≈ 1.208×10⁻23 kg·m/s
λ = h/p = 6.626×10⁻34 J·s / 1.208×10⁻23 kg·m/s
≈ 5.48×10⁻11 m.
4. Using the uncertainty principle ΔxΔp ≥ h/4π, find the minimum momentum uncertainty Δp if Δx = 1.0×10⁻10 m. (h = 6.626×10⁻34 J·s)
Solution:
Δp ≥ h/(4πΔx) = 6.626×10⁻34 / (4π × 1.0×10⁻10)
≈ 6.626×10⁻34 / 1.2566×10⁻9
≈ 5.27×10⁻25 kg·m/s.
5. Calculate the de Broglie wavelength of an electron moving at 2.0×10⁶ m/s. (m = 9.11×10⁻³¹ kg, h = 6.626×10⁻34 J·s)
Solution:
p = m×v = 9.11×10⁻³¹ kg × 2.0×10⁶ m/s = 1.822×10⁻24 kg·m/s
λ = h/p = 6.626×10⁻34 J·s / 1.822×10⁻24 kg·m/s
≈ 3.637×10⁻10 m.
6. For a hydrogen atom, use the Bohr model to calculate the energy difference (ΔE) between the n=2 and n=1 levels. (E_n = -13.6 eV/n²)
Solution:
E₁ = -13.6 eV, E₂ = -13.6/4 = -3.4 eV
ΔE = E₁ – E₂ = (-13.6) – (-3.4) = -10.2 eV
The energy released is 10.2 eV.
7. Calculate the frequency of a photon with energy 3.0 eV using E = hν. (h = 4.1357×10⁻15 eV·s)
Solution:
ν = E/h = 3.0 eV / 4.1357×10⁻15 eV·s
≈ 7.25×10¹⁴ Hz.
8. An electron in a hydrogen atom is in an energy state of -1.51 eV (n=3). What is the wavelength of the photon emitted when it transitions to n=2 (E = -3.4 eV)? (ΔE = 1.89 eV, use E = hc/λ with hc = 1240 eV·nm)
Solution:
λ = hc/ΔE = 1240 eV·nm / 1.89 eV
≈ 656 nm.
9. A quantum system has an energy uncertainty ΔE = 0.1 eV. Estimate the minimum lifetime Δt using Δt ≈ ħ/ΔE. (ħ = 6.582×10⁻16 eV·s)
Solution:
Δt = 6.582×10⁻16 eV·s / 0.1 eV
≈ 6.582×10⁻15 s.
10. If a photon’s wavelength is measured to be 400 nm, what is its momentum? (p = h/λ, h = 6.626×10⁻34 J·s)
Solution:
λ = 400 nm = 400×10⁻9 m
p = 6.626×10⁻34 / (400×10⁻9)
≈ 1.6565×10⁻27 kg·m/s.
11. Determine the kinetic energy (in eV) of an electron with a momentum of 1.0×10⁻24 kg·m/s. (Use E = p²/(2m), m = 9.11×10⁻31 kg)
Solution:
E = (1.0×10⁻24)² / (2 × 9.11×10⁻31)
= 1.0×10⁻48 / 1.822×10⁻30
≈ 5.49×10⁻19 J
Convert to eV: 5.49×10⁻19 J / 1.602×10⁻19 J/eV ≈ 3.42 eV.
12. A quantum system is confined to a region of size 1.0×10⁻9 m. Estimate the minimum energy uncertainty ΔE using ΔE ≈ ħc/Δx, with ħc ≈ 197 eV·nm.
Solution:
Δx = 1.0×10⁻9 m = 1.0 nm
ΔE ≈ 197 eV·nm / 1.0 nm
= 197 eV.
