Radioactivity and isotopes lie at the heart of our understanding of unstable nuclei and their transformation into more stable configurations. These phenomena, first observed through natural emissions from uranium salts, are now fundamental topics in physics and are extensively explored in the domain of modern physics. The emission of alpha, beta, or gamma radiation not only reveals the internal structure of matter but also has profound applications in medicine, archaeology, nuclear energy, and particle research.
To fully understand the basis of radioactivity, one must first investigate the atomic physics framework, including concepts such as the structure of the atom and quantum numbers and electron configuration. Understanding isotopes—atoms with the same number of protons but different numbers of neutrons—helps explain why some nuclei are inherently unstable.
These instabilities and transformations are studied under nuclear physics. Through this lens, we explore not only spontaneous emissions but also interactions like nuclear fission, nuclear fusion, and other nuclear reactions. These processes are key to understanding not just natural decay chains but also energy production in reactors and stars.
The role of condensed matter physics becomes significant when radioactive materials interact with solids or biological tissues, influencing material properties and radiation shielding. Moreover, the probabilistic nature of radioactive decay is described using statistical mechanics, which allows for predictions of half-life and decay rates on a macroscopic scale.
A comprehensive view of radioactivity also involves particle physics. Here, radioactive decay often involves fermions such as electrons and neutrinos, and is mediated by bosons like the W and Z particles. The forces that govern these changes are part of the broader set of fundamental forces studied in high-energy contexts.
Explaining the behavior of radioactive isotopes requires a firm grasp of quantum mechanics. Concepts like Schrödinger’s equation and Heisenberg’s uncertainty principle describe the stochastic nature of decay processes. Quantum effects such as quantum tunneling are central to alpha decay, where particles escape the nucleus despite insufficient classical energy.
Further quantum phenomena like quantum superposition, entanglement, and wave-particle duality offer deeper insight into the probabilistic interpretation of decay chains and energy emissions. Meanwhile, quantum field theory provides a robust mathematical framework for modeling these subatomic events.
Even the role of relativity is relevant, especially when considering the conversion of mass into energy during radioactive transformations, as expressed by Einstein’s equation E = mc². Overall, the study of radioactivity and isotopes is a vibrant field that bridges classical ideas with advanced quantum and particle theories.
Radioactive Isotopes:
The study of radioactive decay and isotopes has led to groundbreaking applications across various fields. In medicine, radioactive isotopes are used in diagnostic imaging (e.g., technetium-99m in nuclear medicine scans) and cancer treatments (e.g., cobalt-60 in radiotherapy). In industry, isotopes are employed for material testing, quality control, and sterilization processes using gamma radiation. In archaeology and geology, radiometric dating techniques, such as carbon-14 dating, allow scientists to determine the age of ancient fossils, rocks, and artifacts. Furthermore, radioisotopes are crucial in space exploration, where nuclear batteries, such as those using plutonium-238, provide long-lasting power for deep-space probes. As research advances, radioactive isotopes continue to play an essential role in scientific discovery and technological innovation.
- Nuclear Physics topics:
- Nuclear Physics – Overview
- Radioactivity & Isotopes
- Nuclear Reactions
- Nuclear Fission
- Nuclear Fusion
Table of Contents
Types of Radioactive Decay:
Radioactive decay occurs in different forms depending on the type of particle or energy emitted. Alpha decay involves the emission of an alpha particle (two protons and two neutrons), which reduces the atomic number and mass of the nucleus. This type of decay is common in heavy elements like uranium and radium. Beta decay occurs when a neutron is converted into a proton (or vice versa), releasing an electron or positron and an antineutrino or neutrino. This transformation alters the atomic number of the element, effectively changing it into a different element. Gamma decay, on the other hand, involves the release of high-energy electromagnetic radiation (gamma rays) without altering the composition of the nucleus. Gamma radiation is often emitted following alpha or beta decay to allow the nucleus to settle into a lower-energy state.
- Alpha Decay (α):
- In alpha decay, an unstable nucleus emits an alpha particle, which consists of two protons and two neutrons. This particle is identical to a helium-4.
- Effect on the Atom: The emission reduces the atomic number by 2 and the mass number by 4, resulting in a new element.
- Example:
Uranium-238 decays into thorium-234 by emitting an alpha particle.
- Characteristics: Alpha particles are relatively heavy and carry a +2 charge. They have low penetration power and can be stopped by a sheet of paper or even the outer layer of human skin but are dangerous if ingested or inhaled.
- Beta Decay (β):
Beta decay occurs in two forms: beta-minus (β⁻) and beta-plus (β⁺) decay.- Beta-minus decay (β⁻): A neutron in the nucleus converts into a proton, emitting an electron and an antineutrino.
Example:
Carbon-14 decays into nitrogen-14, releasing a beta particle (electron).
- Beta-plus decay (β⁺): A proton converts into a neutron, emitting a positron (the electron’s antiparticle) and a neutrino.
Example:
Carbon-11 decays into boron-11 by emitting a positron.
- Characteristics: Beta particles are lighter than alpha particles and can penetrate further, being stopped by materials like plastic or thin metal sheets.
- Beta-minus decay (β⁻): A neutron in the nucleus converts into a proton, emitting an electron and an antineutrino.
- Gamma Decay (γ):
- Gamma decay involves the emission of gamma rays, which are high-energy photons. This occurs when a nucleus, after undergoing alpha or beta decay, remains in an excited energy state and releases energy to stabilize.
- Effect on the Atom: Gamma emission does not change the atomic number or mass number, only the energy state.
- Example:
Cobalt-60 decays into an excited state of nickel-60, which then emits gamma radiation.
- Characteristics: Gamma rays are highly penetrating electromagnetic waves and require dense materials like lead or several centimeters of concrete for shielding.
Half-Life:
The half-life of a radioactive isotope is the time required for half of the atoms in a given sample to decay into more stable forms. It is a statistical measure reflecting the stability of a radioactive isotope. The half-life is constant for any given isotope and is not affected by external conditions such as temperature, pressure, or chemical state.
- Example:
Carbon-14has a half-life of approximately 5,730 years. This property makes it ideal for dating ancient organic materials in archaeology and geology. - Short vs. Long Half-Lives:
- Isotopes with short half-lives (seconds to days) decay rapidly and are useful for medical applications.
- Isotopes with long half-lives (thousands to billions of years) persist in the environment and are useful for dating geological formations.
Isotopes and Their Applications:
Isotopes, both stable and radioactive, have a wide array of applications across various fields due to their unique nuclear properties.
1. Radiocarbon Dating (Carbon-14):
- Used in archaeology and geology to date ancient organic materials.
- Living organisms continuously absorb carbon-14. After death, the carbon-14 decays, and the remaining amount can be measured to estimate the object’s age.

2. Cancer Treatment (Cobalt-60):
- Cobalt-60 emits high-energy gamma rays, which are used in radiotherapy to target and destroy cancerous cells while minimizing damage to surrounding healthy tissue.

3. Medical Imaging (Technetium-99m):
- Technetium-99m is widely used in nuclear medicine for imaging organs and detecting abnormalities.
- Its short half-life (~6 hours) and gamma emission make it ideal for diagnostic scans without prolonged radiation exposure.
4. Sterilization (Cobalt-60 and Cesium-137):
- Gamma radiation from isotopes like cobalt-60 is used to sterilize medical equipment and food by killing bacteria and other pathogens.

5. Smoke Detectors (Americium-241):
- Americium-241 emits alpha particles, which ionize air in smoke detectors. The presence of smoke disrupts this ionization, triggering the alarm.

6. Nuclear Power (Uranium-235 and Plutonium-239):
- Radioactive isotopes are used as fuel in nuclear reactors. Their fission reactions generate massive amounts of heat, which is converted into electricity.
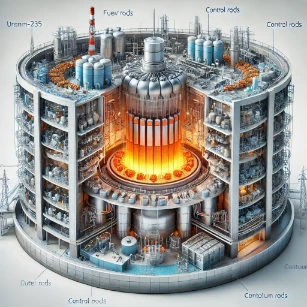
7. Industrial Radiography (Iridium-192):
- Used for non-destructive testing to inspect metal structures, welds, and pipelines for internal defects using gamma rays.

Safety and Environmental Considerations:
While radioisotopes offer immense benefits, their use also comes with safety and environmental challenges:
- Radiation Exposure: Prolonged or intense exposure can damage living tissues, leading to burns, radiation sickness, or increased cancer risk.
- Radioactive Waste: Disposal of radioactive materials, particularly long-lived isotopes from nuclear reactors, requires secure and long-term containment.
- Nuclear Accidents: Accidents like Chernobyl and Fukushima highlight the need for stringent safety measures in handling radioactive materials.
- Environmental Impact: Improper disposal or accidental release of radioactive isotopes can lead to long-term environmental contamination.
Why Study Radioactivity and Isotopes
Spontaneous Nuclear Decay and Stability
Radioactivity is the process by which unstable nuclei emit particles or radiation to achieve greater stability. Students learn about alpha, beta, and gamma decay and the forces that govern nuclear stability. This knowledge provides insight into atomic structure and the limits of nuclear binding. It is fundamental to both theoretical and applied nuclear science.
Half-Life and Nuclear Dating Techniques
Students study half-life as a measure of radioactive decay and use it to analyze long-term processes. This concept underpins techniques such as radiocarbon and uranium-lead dating. It allows scientists to determine the age of fossils, artifacts, and geological formations. It connects physics with archaeology, geology, and environmental science.
Medical and Industrial Applications of Isotopes
Radioactive isotopes are used in diagnostics, therapy, sterilization, and industrial tracing. Students explore how isotopes are selected, produced, and administered safely. This builds knowledge relevant to nuclear medicine and environmental monitoring. It demonstrates the societal value of radioactive technologies.
Safety, Regulation, and Environmental Impact
Handling radioactive materials requires stringent safety protocols and regulatory oversight. Students learn about shielding, contamination prevention, and radiation dose limits. This fosters responsible practices in scientific and technical fields. It emphasizes ethical awareness and risk management.
Link to Nuclear Physics and Beyond
Radioactivity connects nuclear physics with particle physics, medicine, and environmental science. Students gain tools for understanding natural and artificial radiation sources. This knowledge prepares them for interdisciplinary applications and research. It supports a comprehensive view of nuclear science’s role in society.
Radioactivity and Isotopes: Conclusion:
Radioactivity and isotopes play a pivotal role in advancing science, technology, and medicine. From enabling archaeologists to unlock the secrets of ancient civilizations to providing life-saving cancer treatments, radioactive isotopes have countless beneficial applications. However, their inherent dangers necessitate careful handling, strict safety protocols, and responsible use to prevent harmful consequences. Understanding the nature of radioactive decay and the properties of isotopes is essential to harness their potential while minimizing associated risks.
Frequently Asked Questions: Radioactivity and Isotopes
1. What is radioactivity?
Radioactivity is the spontaneous process by which unstable atomic nuclei transform into more stable nuclei, emitting particles or high-energy electromagnetic radiation in the process. This transformation changes the nucleus and often the chemical element itself.
2. What are isotopes?
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons in the nucleus. They occupy the same position in the periodic table but can have different masses and nuclear properties, including stability and radioactivity.
3. What is the difference between stable and unstable isotopes?
Stable isotopes have nuclei that do not undergo spontaneous radioactive decay under normal conditions. Unstable, or radioactive, isotopes have nuclei that can change into other nuclei by emitting radiation. The tendency to decay depends on the balance of protons and neutrons and the nuclear binding energy.
4. What are the main types of radioactive decay?
The main types of radioactive decay are alpha decay, beta decay, and gamma decay. In alpha decay, the nucleus emits a helium-4 nucleus. In beta decay, a neutron can change into a proton (or vice versa), emitting an electron or positron and a neutrino. In gamma decay, the nucleus emits high-energy photons as it moves from an excited state to a lower-energy state.
5. What is half-life and what does it tell us?
The half-life of a radioactive isotope is the time it takes for half of the nuclei in a sample to decay. It is a statistical measure of how quickly a nucleus tends to decay and can range from fractions of a second to billions of years. Half-life is used to characterise isotopes and to plan medical, industrial, and dating applications.
6. How is radioactivity measured?
Radioactivity is measured in terms of activity, dose, and dose rate. Activity, often measured in becquerels, indicates how many decays occur per second. Dose and dose rate, measured in grays or sieverts, describe how much energy from radiation is absorbed by matter, especially living tissue, and are used to assess potential health effects.
7. Where does natural background radiation come from?
Natural background radiation comes from several sources: radioactive isotopes present in rocks, soil, and the human body; cosmic rays from space; and radon gas produced by the decay of uranium in the Earth’s crust. Humans are exposed to a low level of background radiation all the time.
8. How are radioactive isotopes used in medicine?
Radioactive isotopes are widely used in medicine for diagnosis and treatment. In nuclear medicine, tracers are used in imaging techniques such as PET and SPECT to study organ function. In radiotherapy, carefully controlled radiation from isotopes or accelerators is used to damage cancer cells while aiming to spare healthy tissue.
9. How are isotopes used in environmental and archaeological studies?
Isotopes are used as tracers and clocks in many fields. Radiocarbon dating uses the decay of carbon-14 to estimate the age of once-living materials. Other isotopes help trace water flow, study climate history in ice cores, and track pollution sources. Stable isotopes are also used to investigate food webs and migration patterns in ecology.
10. What safety measures help protect people from ionising radiation?
Radiation safety relies on three key principles: time, distance, and shielding. Reducing exposure time, increasing distance from the source, and using appropriate shields (such as lead, concrete, or water) all lower dose. Monitoring devices, safety training, and regulations ensure that workers and the public stay within recommended exposure limits.
11. What is meant by radioactive contamination and how is it different from radiation exposure?
Radioactive contamination refers to the unwanted presence of radioactive material on surfaces, in liquids, or inside the body. Radiation exposure means being subjected to ionising radiation from a source, whether or not contamination is present. You can be exposed to radiation without being contaminated, for example when having an X-ray scan.
12. Why should students learn about radioactivity and isotopes before university?
Learning about radioactivity and isotopes before university helps students understand how nuclei change, how we measure time and processes in nature, and how radiation can be both useful and hazardous. It connects nuclear physics to medicine, energy generation, environmental science, and archaeology, and prepares students for more advanced study in physics, chemistry, and engineering.
Radioactivity and Isotopes: Review Questions and Answers:
1. What is radioactivity?
Answer: Radioactivity is the spontaneous process by which unstable atomic nuclei emit particles or electromagnetic radiation in order to reach a more stable configuration.
2. What are isotopes?
Answer: Isotopes are variants of a chemical element that have the same number of protons but different numbers of neutrons, resulting in different atomic masses and sometimes differing stability.
3. What are the main types of radioactive decay?
Answer: The main types of radioactive decay include alpha decay (emission of helium nuclei), beta decay (emission of electrons or positrons), and gamma decay (emission of high-energy photons), each of which alters the nucleus in a unique way.
4. What is half-life, and why is it important?
Answer: The half-life of a radioactive isotope is the time required for half of the nuclei in a sample to decay. It is a critical parameter for determining the rate of decay, the age of materials, and for applications in medicine and dating techniques.
5. How is radioactivity measured?
Answer: Radioactivity is measured in units such as the becquerel (Bq), which represents one decay per second, or the curie (Ci), which is based on the activity of radium-226, providing a quantifiable measure of radioactive emissions.
6. What factors determine whether an isotope is stable or radioactive?
Answer: The stability of an isotope is determined by the ratio of neutrons to protons in its nucleus. Deviations from the optimal ratio lead to instability, causing the nucleus to decay in order to achieve a more balanced configuration.
7. How does the exponential decay law describe radioactive decay?
Answer: The exponential decay law states that the number of radioactive nuclei decreases exponentially over time, following the equation N(t) = N₀e^(–λt), where N₀ is the initial quantity, λ is the decay constant, and t is time.
8. What role do radioactive isotopes play in medical applications?
Answer: Radioactive isotopes are used in medicine for diagnostic imaging (such as PET and SPECT scans) and for treatment (such as in radiotherapy), providing critical tools for detecting and treating diseases like cancer.
9. How do stable isotopes differ from radioactive isotopes?
Answer: Stable isotopes do not undergo radioactive decay over time, whereas radioactive isotopes are unstable and decay by emitting radiation. This difference affects their applications, with radioactive isotopes being used in imaging and therapy and stable isotopes often used in tracing studies.
10. What are some industrial applications of radioactivity?
Answer: Radioactivity is applied in various industrial fields including material analysis, radiography for non-destructive testing, sterilization of medical equipment, and in techniques such as radiometric dating for determining the age of archaeological finds.
Radioactivity and Isotopes: Thought-Provoking Questions and Answers
1. How could advances in our understanding of radioactive decay improve nuclear waste management?
Answer: Advances in decay modeling and isotopic analysis can lead to improved prediction of long-term waste behavior, enabling the development of more effective storage and disposal methods. Better understanding may also lead to methods for transmuting long-lived isotopes into shorter-lived or stable forms, reducing environmental and health risks.
2. What are the potential benefits and risks of using radioisotopes in medical diagnostics and treatment?
Answer: The benefits include early and accurate disease detection and targeted treatment options that can improve patient outcomes. However, risks involve radiation exposure, potential side effects, and the need for stringent safety protocols to protect both patients and medical staff from harmful doses.
3. In what ways can the study of isotopes contribute to our understanding of climate change and environmental processes?
Answer: Isotope analysis is a powerful tool for tracing environmental processes, such as water cycle dynamics, atmospheric circulation, and carbon cycling. Stable isotopes can serve as natural tracers in climate research, helping to reconstruct past climates and predict future environmental changes.
4. How might emerging technologies enhance the precision of half-life measurements for radioactive isotopes?
Answer: Emerging technologies such as advanced detectors, improved timing electronics, and machine learning algorithms can refine half-life measurements by reducing experimental uncertainties. This increased precision benefits applications ranging from nuclear medicine to geochronology, where accurate dating is essential.
5. What ethical considerations should be taken into account when using radioactive materials in research and industry?
Answer: Ethical considerations include ensuring the safe handling, storage, and disposal of radioactive materials to protect human health and the environment. There is also a responsibility to manage nuclear technology to prevent its misuse, ensure transparency in research, and minimize the potential for accidents or environmental contamination.
6. How do differences in neutron-to-proton ratios among isotopes influence their chemical behavior and applications?
Answer: The neutron-to-proton ratio affects the nuclear stability of an isotope, but it can also subtly influence atomic mass and bonding characteristics. In some applications, such as isotope labeling in chemistry and biology, these differences are exploited to trace molecular pathways without altering chemical properties significantly.
7. What challenges exist in the production and purification of medical radioisotopes, and how can they be overcome?
Answer: Challenges include producing sufficient quantities with high purity, short half-lives requiring rapid processing, and the need for specialized facilities. Overcoming these challenges involves advances in reactor design, accelerator technology, and chemical separation techniques to efficiently produce and purify isotopes for clinical use.
8. How can radiometric dating techniques be improved to provide more accurate historical and geological timelines?
Answer: Improvements in detector sensitivity, calibration methods, and error reduction in isotope ratio measurements can enhance the accuracy of radiometric dating. Combining multiple dating methods and using high-precision mass spectrometry further refines age estimates, providing clearer insights into historical and geological events.
9. What potential does isotope research hold for the development of new energy sources?
Answer: Research into isotopes can lead to the discovery of new materials for nuclear batteries or the optimization of fuel cycles in nuclear reactors. Isotopic separation and enrichment techniques are crucial for developing advanced reactors that use alternative fuels, potentially offering safer and more sustainable energy solutions.
10. How might improved understanding of radioactive decay influence safety protocols in nuclear power plants?
Answer: A deeper understanding of decay processes can lead to better prediction models for radiation levels and waste heat, enabling more effective design of shielding, cooling systems, and emergency response plans. This contributes to enhanced safety measures and more efficient operation of nuclear facilities.
11. In what ways could isotope geochemistry revolutionize our understanding of Earth’s formation and evolution?
Answer: Isotope geochemistry allows scientists to trace the origins and movements of materials within the Earth, providing insights into mantle convection, crust formation, and the timing of geological events. These studies can reveal patterns of planetary differentiation and offer clues about the processes that shaped our planet.
12. How can interdisciplinary collaboration between nuclear physicists, chemists, and environmental scientists lead to innovative applications of radioisotopes?
Answer: Interdisciplinary collaboration fosters the integration of diverse expertise, leading to innovative solutions in areas such as environmental monitoring, medical diagnostics, and resource exploration. By combining knowledge of nuclear reactions, chemical behavior, and environmental dynamics, researchers can develop novel techniques and applications that benefit multiple fields.
Numerical Problems and Solutions
1. Calculate the half-life of an isotope if its decay constant is 2.5×10⁻⁷ s⁻¹.
Solution:
Half-life, t₁/₂ = ln(2) / λ = 0.693 / (2.5×10⁻⁷) ≈ 2.772×10⁶ s.
2. A sample of a radioactive isotope has an activity of 1.0×10⁶ Bq. If the decay constant is 1.0×10⁻⁵ s⁻¹, determine the number of radioactive atoms in the sample.
Solution:
Number of atoms, N = Activity / λ = 1.0×10⁶ / 1.0×10⁻⁵ = 1.0×10¹¹ atoms.
3. Convert 150 Curie to Becquerel. (1 Ci = 3.7×10¹⁰ Bq)
Solution:
Activity in Bq = 150 Ci × 3.7×10¹⁰ Bq/Ci = 5.55×10¹² Bq.
4. A radioactive isotope decays following the law N(t) = N₀e^(–λt). If a sample starts with 8.0×10¹² atoms and 2.0×10¹² atoms remain after 10 days, calculate the decay constant λ.
Solution:
2.0×10¹² = 8.0×10¹² × e^(–λ×(10×86400))
Divide: 0.25 = e^(–λ×864000)
Taking natural logs: ln(0.25) = –λ×864000 → –1.386 = –λ×864000
λ = 1.386 / 864000 ≈ 1.603×10⁻⁶ s⁻¹.
5. Determine the energy in joules of a gamma ray with a frequency of 2.0×10²⁰ Hz. (Planck’s constant, h = 6.626×10⁻³⁴ J·s)
Solution:
Energy, E = hν = 6.626×10⁻³⁴ × 2.0×10²⁰ = 1.3252×10⁻¹³ J.
6. If an isotope has a half-life of 20 years, what fraction of the original sample remains after 60 years?
Solution:
Number of half-lives = 60 / 20 = 3.
Remaining fraction = (1/2)³ = 1/8 = 0.125.
7. A radioactive decay releases 5.0 MeV per event. How many decay events are required to release a total of 1.0×10¹² J? (1 MeV = 1.602×10⁻¹³ J)
Solution:
Energy per event = 5.0 × 1.602×10⁻¹³ = 8.01×10⁻¹³ J.
Number of events = 1.0×10¹² J / 8.01×10⁻¹³ J ≈ 1.25×10²⁴ events.
8. Calculate the decay constant of an isotope with a half-life of 8.0 days. (1 day = 86400 s)
Solution:
Half-life in seconds = 8.0 × 86400 = 691200 s.
λ = 0.693 / 691200 ≈ 1.002×10⁻⁶ s⁻¹.
9. A medical isotope is administered with an initial activity of 2.0×10⁷ Bq. If its half-life is 6 hours, what will be its activity after 18 hours?
Solution:
Number of half-lives = 18 / 6 = 3.
Activity = 2.0×10⁷ Bq × (1/2)³ = 2.0×10⁷ / 8 = 2.5×10⁶ Bq.
10. A radioactive sample decreases from 5.0×10¹² atoms to 1.25×10¹² atoms in a certain time period. How many half-lives have passed?
Solution:
5.0×10¹² / 1.25×10¹² = 4, so 4 = 2ⁿ, where n = 2.
Thus, 2 half-lives have passed.
11. Convert an activity of 0.75 Ci to Bq. (1 Ci = 3.7×10¹⁰ Bq)
Solution:
Activity = 0.75 × 3.7×10¹⁰ = 2.775×10¹⁰ Bq.
12. If a radioactive isotope has a decay constant of 4.0×10⁻⁷ s⁻¹, how long (in years) will it take for 90% of the sample to decay?
Solution:
We need t such that N/N₀ = 0.10 = e^(–λt).
Taking ln: ln(0.10) = –λt → t = –ln(0.10) / λ = 2.3026 / 4.0×10⁻⁷ ≈ 5.7565×10⁶ s.
Convert seconds to years: 5.7565×10⁶ / (3.156×10⁷) ≈ 0.1825 years.
